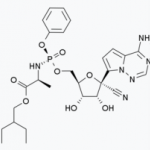
Remdesivir, an antiviral drug that many are pinning their hopes on to help solve this pandemic nightmare, is now being tested in hundreds of trials. Results are expected within weeks. But the drug has already been tested in monkeys. And it worked.
Drugs & Pharmaceuticals
The seasonality of coronavirus infections is the basis of hope. If COVID-19 goes on until July – as it well may – it will be disastrous for world health and for the global economy. But, if it is like SARS, it may then die out and not come back. Is this wishful thinking? Yes, but based on scientific fact. Should we hope for the best but prepare for the worst? Absolutely.
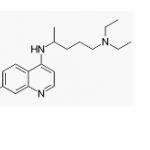
If there was any effect of this drug on COVID-19, it was minimal. Hydroxychloroquine, whose toxicity is far lower, may be safer than chloroquine. But that doesn't matter if the drugs are ineffective.
Chloroquine, the old malaria drug, is making news as a potential therapy for coronavirus. Does it belong in the headlines for its antiviral properties, or is it just hype and bluster? Will it become a drug? Let's find out.
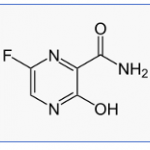
As the horror known as the coronavirus tightens its grip on the world, and a vaccine is years away, our best hope is an antiviral drug that minimizes the damage caused by coronavirus replication. New data on favipiravir, a repurposed drug originally discovered in Japan, looks promising in trials in China. But nothing is ever straightforward in drug discovery -- and that is no different here. Here's a summary of the new findings.
As the Wuhan coronavirus relentlessly engulfs the world, scientists are relentlessly looking for a way to treat the infection. A vaccine is more than a year away, but an antiviral drug called remdesivir is being evaluated in clinical trials by Gilead Science, the world's premier antiviral drug company. Keep your fingers crossed.
The job of a pharmacist is as tough as ever, and the term "pharmacist burnout" is now become common. ACSH advisor Dr. Jeff Singer has an intriguing solution: Using vending machines to dispense certain prescription drugs. What do you think? Check this out.
As the coronavirus continues to terrorize the world, people are pinning their hopes on companies that are doing vaccine and drug research to -- maybe -- get us out of this mess. Yet, many of the companies doing the work, especially Gilead Science, are "the bad guys." Except when we need them. Gilead's drug, remdesivir, is now in clinical trials in China so they're OK for now. Hypocrisy at its finest.
At first glance, rheumatoid arthritis and coronavirus have little in common. But an underlying pathological mechanism that involves an over-reactive immune response may allow a drug developed to treat arthritis to save the lives of coronavirus victims.
When I wrote about "Magic Aleve" -- a derivative of Aleve/naproxen that appears to be both G.I.-friendly and a more potent analgesic/antiinflammatory than Aleve itself -- a number of questions arose. Dr. John Wallace, CSO of Antibe, which is developing the drug called ATB-346, kindly agreed to answer them.
For patients taking multiple medications, one extended-release pill increases their compliance in taking the medication, as compared with two immediate-release pills. So if we want to encourage compliance, why are extended-release pills more expensive than what they replace?
There hasn't been a material advance in the pharmacological treatment of pain since the 1890s, when heroin and aspirin were invented. That may change if an experimental drug being developed by a Toronto-based drug company keeps performing in advanced clinical trials. This could be huge.