Welcome to the 15th Dreaded Chemistry Lesson From Hell. This time you will be (mis)treated to a plethora of knowledge about bismuth - information so critical that it would be hard to imagine surviving the day without it. And reader comments from Joe, the ball bearing salesman, and Candy, a decertified yoga instructor. Could there be a better way to start your day?
Not that I'm counting, but this is my 15th Dreaded Chemistry Lesson From Hell®
Reader reactions have been mixed...
"You suck. Your chemistry sucks. And your car sucks."
Joe, a ball bearing salesman from Toledo, OH
But there's this too...
"I just LUV your chemestry lessons! And your really cute! xoxoxoxo"
Candy, a decertified yoga instructor from Venice Beach, CA
But, for the most part, losers readers seem to like them, something that continues to baffle me three years after I first tried this stupid idea.
So, let's do another one about bismuth, a rather strange element with some VERY bad neighbors.
These days, If you're engaged in a conversation the word "bismuth" comes up – admittedly unlikely –better make sure you have your mask on. Try saying the word without having saliva fly out of your mouth. It's impossible. Not only will your conversation hostage partner have to listen to mind-bending tedium but may also come down with COVID in the process. Not sure which is worse.
1. BISMUTH LIVES IN A BAD NEIGHBORHOOD
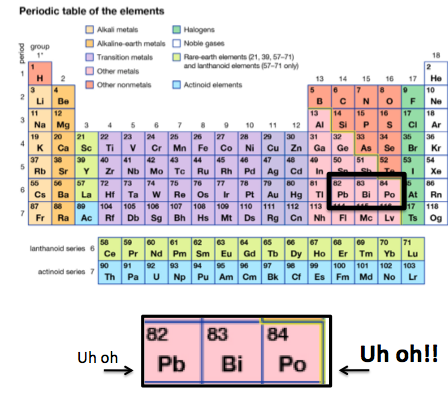
Bismuth is a metal surrounded by lead and polonium, both toxic. We all know about lead, but polonium, which I'll write about another time, is a real doozie. It has a number of isotopes, all radioactive, but the isotope 210Po is infamous, as it was implicated in the murder of a former Russian spy (See the BBC article "Alexander Litvinenko: Profile of murdered Russian spy.")
2. BISMUTH IS SORT OF UGLY BUT IT CAN ALSO BE BEAUTIFUL

Bismuth metal. Meh. Photo: Wikipedia
But it can also look like this:

When bismuth is left in air it reacts with oxygen to give a thin film of bismuth oxide. The oxide has light-scattering properties, which are responsible for the colors. The colors are dependent on the thickness of the film. Wow! (Photo: Wikipedia)
3. BISMUTH HAS MEDICAL USES. ONE IS OBVIOUS, THE OTHER IS NOT.
Who amongst us hasn't gagged down a mouthful of bismuth subsalicylate, better known as Pepto Bismol for heartburn, nausea, and diarrhea? Although, in my experience, it's not entirely clear whether it treats these maladies or causes them. Although bismuth, like most metals, has some toxicity bismuth subsalicylate has little or none (1). This is because the chemical is so insoluble in water that I couldn't even find solubility data other than "insoluble in water."
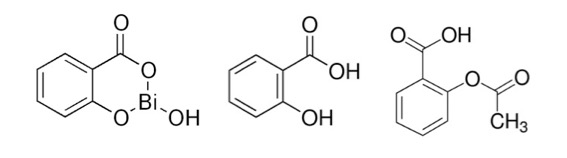
Bismuth subsalicylate (left) is a complex of salicylic acid (middle) and bismuth oxide. Note the structural similarity to aspirin (acetylsalicylic acid, right). This is the reason that people who are allergic to aspirin may have problems with Pepto Bismol.
Finally, I was surprised to find that there is another use for bismuth. High doses of bismuth nitrate can alleviate some of the toxicity of cisplatin – arguably the worst chemotherapy drug you'll ever encounter.
You've probably had (more than) enough chemistry for one decade day, so I'll close with this. Have you ever taken Pepto Bismol and had your tongue (or stools) turn black? This is due to another chemical reaction. Bismuth reacts with sulfides, for example hydrogen sulfide) in your mouth to form bismuth sulfide, which is black.

Bottoms up!
NOTE
(1) Even though bismuth subsalicylate is very safe because of its insolubility, there have been rare cases of severe bismuth poisoning as well as allergic reactions to the salicylate part, especially in people who are allergic to aspirin.

