When the universe formed, there was hydrogen, helium with a light smattering of lithium – every atom in the universe heavier than a lithium atom was created inside a star. Stars like our Sun can produce atoms up to about the mass of iron. Every heavier atom formed in one of the rare stars heavy enough to end their lives in the titanic explosion of a supernova.
About six or seven billion years ago, a massive star exploded, and its debris, containing (among other things) traces of gold, mercury, lead, and uranium, slammed into a cloud of gas and dust floating on the outskirts of the galaxy. The impact caused the cloud to start to collapse, ultimately forming a star and a cluster of planets – including Earth.
The early Earth was well-mixed, and uranium was found in many locations. Over the eons, though, most of the planet’s iron and nickel sank through the rock to form our core. At the same time, larger atoms (including uranium) became increasingly partitioned into the crust – by about 2.5 billion years ago, the Earth had more or less the layers, core, mantle, and crust and elemental concentrations we see today.
There the uranium sat for another half-billion year – waiting for oxygen.
It turns out that uranium is quite soluble in oxygenated water but not in the anoxic waters of the early Earth. For the first few billion years of Earth’s existence, all of the oxygen produced by cyanobacteria was absorbed by the metals in the crust – the uranium couldn’t go anywhere until the planet rusted.
Once oxygen began accumulating in the atmosphere, the rain could wash uranium out of the igneous rocks. The rivers could carry it downstream, and it could start to accumulate in swamps and other places in which decaying organic material sucked the oxygen out of the water.
One such location was today’s nation of Gabon in West Africa. In the 1970s, French geologists located a remarkably rich uranium ore deposit that they began mining, but when they fed the uranium into their enrichment cascade, the output was not as enriched as it should have been. Everywhere on Earth, there are 992 U-238 atoms to seven atoms of more fissile, and therefore more useful, U-235…except in this one ore deposit in Gabon, which had only half the expected U-235. Why?
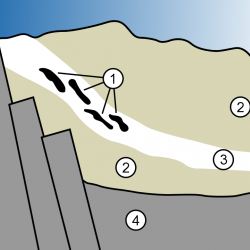
In May, I wrote an article about how nuclear reactors work; here’s the short version. A reactor consists of uranium fuel surrounded by water – the fast neutrons given off by a fissioning uranium atom are slowed down (moderated) by the water to the point where they can cause additional atoms to fission, creating a self-sustaining “chain reaction” – the reactor is said to be “critical.” The uranium deposits in Oklo, Gabon were in sandstone, a porous rock. The sandstone laying below the water table was saturated with water which served to moderate neutrons from fission, allowing the lumps of uranium to achieve criticality – it became a natural nuclear reactor.
So that’s the “how” – but why isn’t Earth littered with natural reactors today. How is it that natural uranium could achieve criticality in Oklo, but we have to enrich uranium today? Physics again provides an answer.
That distant supernova that created the Earth’s uranium produced almost equal quantities of U-235 and U-238, not the paltry 0.72% of today’s U-235 atoms. The thing is, U-235 decays more rapidly than does U-238; the half-life of U-238 is a sedate 4.4 billion years while U-235 is a much shorter 704 million years. Six billion years ago, both isotopes were present in roughly equal concentrations, but because of those very different rates of decay, less than 1% of today’s natural uranium is U-235.
When the Oklo “reactor” was operating, slightly more than 3% of natural uranium consisted of U-235 – roughly comparable to uranium enrichment in today’s nuclear reactors. The Oklo reactor formed during a brief window of time, geologically speaking. There was enough oxygen to allow uranium to mobilize and collect in mineral deposits, and there was still enough U-235 to sustain a critical chain reaction.
As the uranium fissioned, it produced heat (as do our reactors today), and as the heat built up, it boiled the water that was moderating the chain reaction. When this happened, the reaction shut down until the rocks cooled enough that water could again saturate the formation. It appears as though Oklo operated in this fashion for about 100,000 years – not long compared to the history of the Earth, but a significant chunk in the history of humanity.
Radioactive Waste
Of course, when uranium fissions, it produces radioactive fission products and forms transuranic elements such as plutonium, some of which have half-lives of several millennia – these are what raise so many concerns about the long-term disposal of radioactive waste. What’s interesting here is a study published in 1989 showing that a surprising percentage of fission products from the Oklo reactors were retained in the reactor zones or in the rock formation that hosted them, even after nearly two billion years. This is in spite of the fact that this rock formation wouldn’t meet today’s standards for a radioactive waste disposal site because it’s in porous rock that’s been fractured and submerged by groundwater for most of its history. This bodes well for waste disposed of in an engineered waste repository that’s dry, monitored, and set in more acceptable rocks.
One last word about Oklo seems relevant.
The conditions that led to its formation – oxygen levels, the presence of uranium, sedimentary basins in which uranium-bearing water could deposit uranium in anoxic areas, and so forth – were not rare at the time that the Oklo reactor formed. It is entirely possible that multiple natural reactors formed during the aforementioned geologic window and that the traces have been eroded, covered with lava, or obliterated by plate tectonics. Oklo itself might not be unique – it could be that the great novelty is its preservation to the present. But no matter how we look at it, Oklo is both humbling and reassuring. Humbling to know that nature managed to produce a nuclear reactor nearly two billion years before humans even walked the Earth. And reassuring to know that the significant problems of long-term radioactive waste disposal might not be nearly the problem we think.