A new JAMA Open Network paper concludes that Paxlovid is effective in reducing hospitalizations and deaths in high-risk patients who have been vaccinated or have acquired immunity from previous infections. And a look back at how the drug works its "magic."
We are approaching the two-year anniversary of the Emergency Use Authorization (EUA) of Paxlovid, the most effective COVID-19 antiviral drug. A new paper from the Cleveland Clinic, which appears on the JAMA Open Network site, examines the utility of the drug (1) in "real life." For example, clinical trials used to support Pfizer's EUA request were conducted on people who had not been vaccinated, which raised questions about the efficacy of the drug at this time when most people have already been infected with COVID-19, been vaccinated, or both.
Some highlights include:
- The EUA trials were conducted at a time (during the deadly Delta phase) when immunity from vaccination or prior infection was limited.
- The new (observational) study was carried out during the Omicron phase, where immunity was common, either acquired or from vaccination.
- Primary outcome: time to death following COVID diagnosis. Secondary outcome: the time to hospitalization or death,
- All participants either received at least one COVID-19 vaccine or had a confirmed infection.
- Inclusion criteria were those (aged 12 or older) considered to be at high risk for developing severe disease.
The following graph (cumulative deaths) speaks for itself.
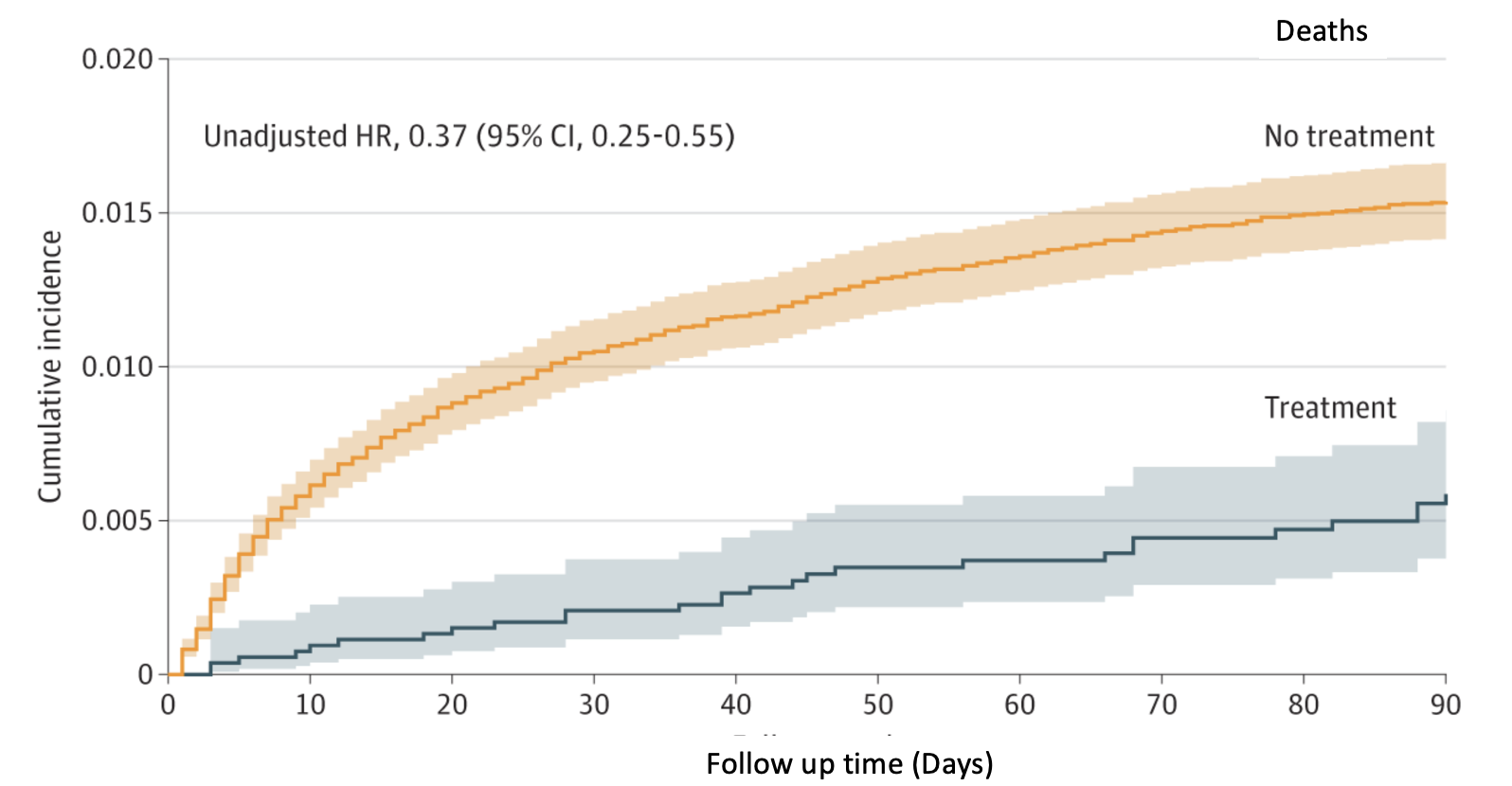
- Original Source: JAMA Netw Open. 2023;6(9):e2335077. doi:10.1001/jamanetworkopen.2023.35077
- The drug seemed less effective (adjusted hazard ratio 0.59) in preventing either hospitalization or death, but these data may be skewed by certain variables in the collected data.
Given these (and other) data, it can reasonably be assumed that Paxlovid is an effective antiviral drug that protects people from severe disease and death.
How does it work?
The following section was written in 2021 – prior to the granting of the EUA. Now that Paxlovid is out and working in the "real world" for nearly two years it is worth taking another look at the science. But the science is fairly heavy. It's about how the drug was designed to inhibit COVID-19's main protease (Mpro), a critical enzyme for viral replication, and why it does so. Tread carefully.
Pfizer's COVID Drug Works Wonders. Here's How It Works (2021).
Before we get started let me warn you that what follows is a big-time chapter in...

Steve (left) and Irving, your hosts of the Dreaded Chemistry Lesson From Hell® are even crankier than usual. Which is a high bar, indeed.
Although different chemists may have other opinions, to me, the most important of all chemical bonds is an amide bond. It consists of a nitrogen atom connected to a carbon atom, which is doubly bound to oxygen (Figure 1).
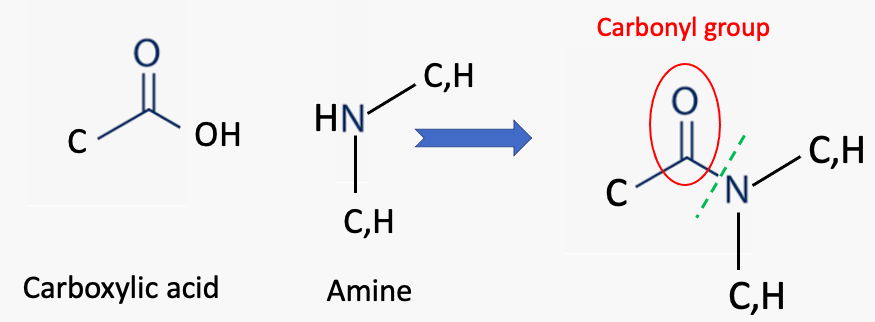
Figure 1. (Left) An amide group consists of a carbon atom (2) bonded to a carbonyl group (red oval), which is connected to a nitrogen atom. The nitrogen atom can be bonded to either carbon or hydrogen atoms.
Things look a little different, but the concept is the same for proteins; the difference is that the amide bond is formed between the amine of one amino acid and the carboxylic acid of another one (Figure 2).
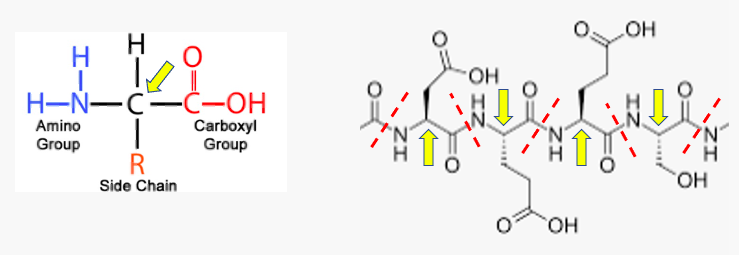
Figure 2. The basic structure of all amino acids that make up proteins. (Left) Amino acids, as the name implies, contain both amine and carboxylic acids in the same molecule. There are 20 different amino acids that make up proteins, all differing only by the side chains. Amino acids are biologically important because they contain both amine and carboxylic acid groups, enabling them to react with themselves to form polymers. These polymers are proteins – the basis of life. (Right) A small fragment of a protein containing four amino acids. The amide bonds are indicated by red hatch marks. The yellow arrows indicate the carbon where the side chains are attached. The largest protein in the human body contains 28,000 amino acids. Image (left): Astrochem
What does this have to do with COVID, Pfizer, or anything else?
This is where it gets interesting. When viruses replicate, they must make copies of their genetic material and manufacture the necessary proteins to provide structure and function for new virus particles. Thousands of amino acids are converted into large proteins by cellular ribosomes – protein factories. Although the ribosomes are perfectly happy to do this job (as if the dumb bastards have anything better to do), they can't finish the job.
When the ribosome makes viral protein, it comes out as one long strand called a polyprotein. Polyproteins are useless to the virus. Although they contain all the structural and enzymatic proteins necessary to make new viruses, they must first be "processed" – cut into smaller pieces, which the virus can then use to replicate. This processing is done by enzymes called proteases. Pfizer's Paxlovid (3) inhibits this process, which is why the drug works; it is a direct-acting antiviral drug (DAAD) that is an inhibitor of the Mpro enzyme that cuts up the COVID polyprotein, allowing viral replication to take place.
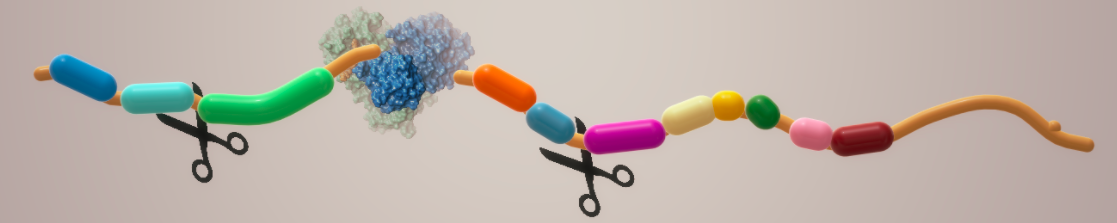
Depiction of a protease at work. The scissors represent the enzyme breaking amide bonds of a polyprotein, forming smaller (and useful) proteins. Image: Biova
One property of amides is that they're chemical tough guys; breaking the carbonyl-nitrogen bond is difficult. The reaction to break this bind is called hydrolysis, and you have to really pound on the molecule to make it happen. For example, conditions required to hydrolyze an amide include boiling it with strong acids (hydrochloric or sulfuric) or strong bases (sodium hydroxide) for hours to complete the reaction; protease enzymes can perform the same reaction millions of times faster. This is why drugs that inhibit these enzymes so effectively shut down polyprotein cleavage and, therefore, viral replication.
Pfizer's drug loves sulfur
There are four different types of protease enzymes. If I try to explain all of this, the two of you who are still reading this hideous article will cheerfully hurl yourselves in front of a bullet train. Since I don't want to lose such a large percentage of my followers, I'll keep it short and simple.
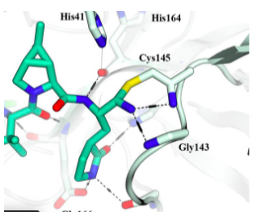
The COVID Mpro (main protease) is a cysteine protease, meaning it uses the thiol (S-H) group on the amino acid cysteine to catalyze the cleavage of the protein's amide bond. Paxlovid is designed to inhibit this process by reacting with the carbon designated with a green star (below).
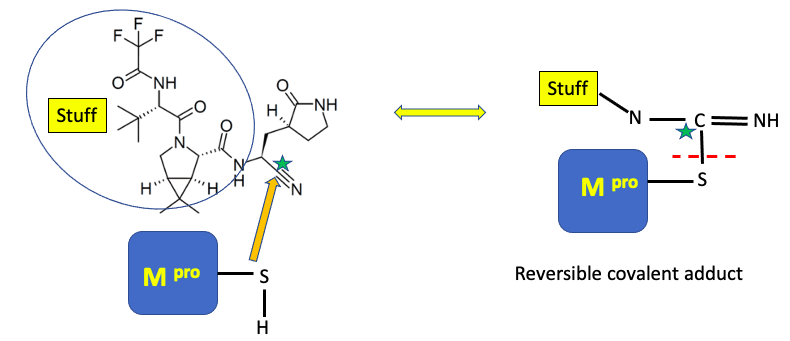
What's going on here is not as bad as it seems. The sulfur of the main protease reacts with (orange arrow) the nitrile (CN) group (green star) of the drug. Without going into too much chemistry, the nitrile is both chemically reactive and held within easy bonding distance of the cysteine sulfur atom (4). This results in the formation of what's called (sorry) a reversible covalent adduct (right), which means that a covalent bond, albeit a reversible bond, is formed between the inhibitor and the enzyme – a very good way to deactivate enzymes. A red hatch line designates this reversible bond. So, while Paxlovid doesn't permanently deactivate Mpro, it does a damn fine job tying it up for a while.
I've sort of disrespected the rest of the molecule (blue oval) by calling it "stuff." But the stuff is also important; it causes Paxlovid to bind to Mpro and places the nitrile (cyano) group in the correct position to entice the enzyme's cysteine group into bonding with it. It wouldn't be inaccurate to call the "stuff" a "molecular wingman."
Bottom line
It is no accident that Paxlovid works; it is designed to do so. This is how drug design works: define an essential viral target (Mpro) and build a molecule to slow it down. This combination of chemistry and enzymology is both simple and complex at the same time and a fine example of how a drug can be rationally designed.
NOTES:
(1) Molnupiravir (Lagevrio), the other approved Covid drug (an inhibitor of the Covid RNA polymerase enzyme), was also discussed in the same JAMA paper. It is beyond the scope of this article.
(2) There is one exception – hydrogen can replace carbon. These amides are called formamides.
(3) Paxlovid is actually a two-drug combination: PF-07321332, the actual inhibitor, and ritonavir, originally an HIV protease inhibitor that inhibits the CYP enzymes that metabolize PF-07321332, giving it a longer half-life. For the sake of simplicity, I'm using the brand name.
(4) If you really want to torture yourself, here is the x-ray structure from a Pfizer paper, which proves the mechanism of action. Although it makes sense, good luck figuring it out.