Long COVID has long been a syndrome in search of a disease and, more importantly, an underlying explanation. A new study in Cell tries to use Occam’s Razor to find the underlying cause. While not truly causal, it is an interesting hypothesis – with lots to unpack.
Long COVID joins other “post-viral” syndromes, ill-characterized by fatigue, a brain fog, and other symptoms perhaps best described as “my get up and go, got up and went.” Hypothetical physiologic “causes” include a persisting cryptic “viral reservoir” with an autoimmune response creating further tissue damage – a version of chronic inflammation. The new study reported in Cell suggests that an underlying lack of serotonin is a causative factor. To arrive at that tentative conclusion, the researchers used various data, including the blood values of patients and experimental findings in rodents and organoids of our gut. [1]
The paper is a beautiful example of how one question leads to another and how a series of experiments, in turn, provides a chain of evidence.
The chain of evidence
- Using the roughly 1,500 participants in a study of the Postacute sequelae of SARS-CoV-2 infection (PASC), the medical name for Long COVID, the researchers identified eight key features of PASC that reflected the disease’s course or trajectory for an individual patient: cardiorespiratory problems, impairment of mobility, fatigue, and neurocognitive changes mixed and matched as depicted below.
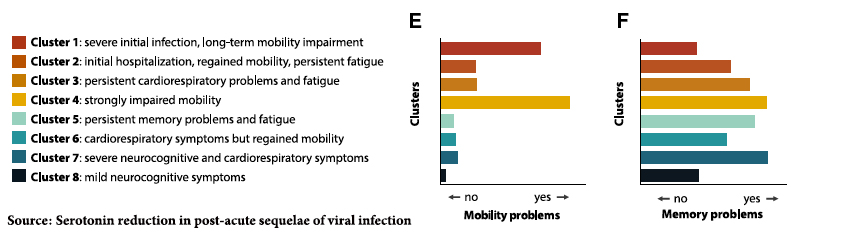
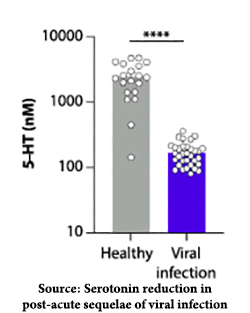 Using 58 individuals representing those 8 clusters along with 60 individuals with acute COVID and 30 who recovered without sequelae, the researchers looked at individual metabolites in their blood. Serotonin (5HT) was deficient in those with acute and post-acute COVID, and an individual’s post-infection serotonin levels predicted who was fully recovered or developed PASC.
Using 58 individuals representing those 8 clusters along with 60 individuals with acute COVID and 30 who recovered without sequelae, the researchers looked at individual metabolites in their blood. Serotonin (5HT) was deficient in those with acute and post-acute COVID, and an individual’s post-infection serotonin levels predicted who was fully recovered or developed PASC.- These post-infection reductions in serotonin were a feature of post-viral infections from viruses other than COVID found in other human subjects. The depletion seemed to result from one component of our immune response, Type 1 Interferon. You may remember Type 1 Interferon by another name; it is a cytokine, one of several that gives us “flu-like” symptoms, and was part of COVID’s “cytokine storm.”
- The bulk of serotonin is produced in our gut synthesized from our dietary intake of tryptophan, an amino acid that we most often wrongly identify with the sleepiness experienced after a Thanksgiving meal. Serotonin is created in enterochromaffin cells that line the intestine and interact with our nervous system – they are the most prolific members of our intestinal enteroendocrine cells, the workers of the “gut-brain” connection. Fun fact: they produce hormones like GLP-1, the underlying mechanism of Ozempic. Through a series of experiments on mice, the researchers demonstrated that the depletion in serotonin was due to viral inflammation altering genetic pathways and reducing serotonin absorption.
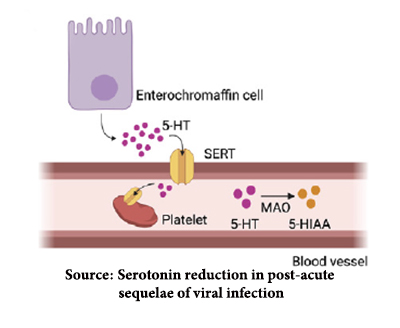 Serotonin, once created, is taken up by platelets, the cellular component that initiates clotting. “Free serotonin,” found in the blood, is rapidly broken down. A reduction in platelets is another hallmark of viral infections generally. Again, using mice models, the researchers demonstrated that viral infection resulted in platelet hyperactivity that, in turn, created clots (hypercoagulability) and depletion of the number of platelets (thrombocytopenia). The development of microclotting has been a recognized hallmark of COVID infections.
Serotonin, once created, is taken up by platelets, the cellular component that initiates clotting. “Free serotonin,” found in the blood, is rapidly broken down. A reduction in platelets is another hallmark of viral infections generally. Again, using mice models, the researchers demonstrated that viral infection resulted in platelet hyperactivity that, in turn, created clots (hypercoagulability) and depletion of the number of platelets (thrombocytopenia). The development of microclotting has been a recognized hallmark of COVID infections. - Finally, the researchers demonstrated that mice experienced cognitive impairments in the presence of both the Type I Interferon signaling that led to and the actual depletion of serotonin. Our short-term memories are “produced” by serotonin in the hippocampus part of the limbic system, which regulates motivation, emotion, learning, and memory. Alterations of hippocampal function have been demonstrated in COVID patients, but the researchers found no evidence of diminished serotonin in the brain. Since serotonin does not cross the blood-brain barrier, they turned their attention to another limbic system component, the vagus nerve, which acts as the neurologic highway between the gut and the brain. Depletion of serotonin dampens the activity of the vagus, which in turn reduces hippocampal activity and may be the causal pathway for the brain fog and other cognitive changes of PASC.
Much of our understanding of the persistence of COVID or its components comes from our study of COVID’s acute phase when it is infectious to others. There is data showing that it can persist for several weeks beyond its infectious stage. Other viral infections, notably HIV and Hepatitis B and C, can persist chronically. So, it is not inconceivable that an indolent COVID infection may underlie PASC, but we have no data.
There are two more tantalizing questions coming out of this work.
First, in mice, using serotonin precursors or SSRIs (selective serotonin uptake inhibitors) that increase serotonin levels in the brain reverses cognitive changes. If the researchers’ hypothesis holds, this may be a part of treating PASC, which, so far, has not had definitive, effective care.
Second, there is some generalizability to this work. Several non-viral diseases with neurocognitive changes, specifically multiple sclerosis and systemic lupus erythematosus, also experience elevated levels of Interferon and low levels of serotonin
“suggest[ing] that the pathway described in this study may even apply beyond viral infections.”
There are limits to what we might conclude; most importantly, PASC remains ill-defined, and the work done here speaks to a possible mechanism for the neurologic symptoms and enhanced clotability. More work needs to be done, but for those who may have PASC, a better understanding of its cause will hopefully lead to more effective care.
In the meantime, as my colleague Dr. Miller often points out, the best defense against PASC is not getting a severe case of COVID. Vaccination and anti-virals remain the only known pathways to reduce severity.
[1] An organoid is a three-dimensional, miniaturized, and simplified version of an organ or tissue grown from stem cells that mimic but do not necessarily replicate the structure and function of our organs.
Source: Serotonin reduction in post-acute sequelae of viral infection Cell DOI: 10.1016/j.cell.2023.09.013

