Sickle Cell Disease is an awful genetic disease that disproportionally affects black people. It's caused by a single-point mutation in DNA, which results in a modified hemoglobin protein, differing by only one amino acid. While this may sound insignificant, it's anything but. Simple organic chemistry explains why this change profoundly affects those unfortunate enough to inherit the disease, which is characterized by abnormal hemoglobin.
Recently, my colleague, Dr. Henry Miller, wrote about the possible use of gene therapy (1) to treat the 1 in 100,000 unfortunate individuals (mostly black) who suffer from sickle cell anemia, aka sickle cell disease (SCD) – an awful condition that changes the shape of red blood cells so that they cannot flow properly through blood vessels. Instead, the blood cells get stuck in vessels where they cause excruciating pain, infection, stroke, and arthritis.
SCD is a genetic condition in which one single amino acid (out of 574) in hemoglobin – the protein responsible for transporting oxygen around the body – is replaced by another. In this case, a single genetic mutation causes the amino acid valine (molecular weight 117) to be replaced by glutamic acid (MW 147) – a difference of 30 atomic mass units in a protein with a molecular weight of 64,000. You might think that a tiny difference in the molecular weight (0.047%) between the normal and mutant forms of hemoglobin would be insignificant. You would be wrong.
I first became aware of SCD during the 1960s because my MIT undergraduate advisor, Professor Vernon Ingram, had unraveled its molecular basis: a mutation in DNA that causes a change in a single amino acid in the hemoglobin molecule...The change occurs specifically at one site where normal hemoglobin has an amino acid called glutamic acid; those with SCD have valine, instead.
Henry Miller, M.D. A Revolutionary New Gene Therapy Treatment For Sickle Cell Anemia Is Imminent
Interesting stuff. How can this be? So, I asked Henry if there was an explanation on the molecular level for the profound change in the red blood cells due to mutated hemoglobin. He didn't know (never ask a physician about chemistry), so I looked it up. Indeed, there is an explanation, and it provides an incredible example of how something taught in Organic 101 can cause such misery: Like dissolves like. First, let's look at why the valine residue (2) replaces glutamic acid: The genetic code.
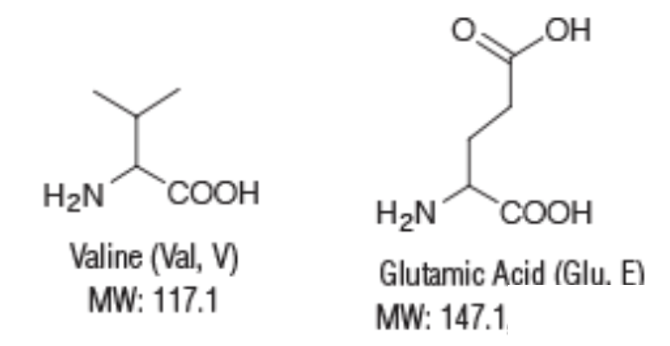
The structures and molecular weights of valine and glutamic acid.
Genetic code 101
Don't let Figure 1 scare you. It's actually rather simple.
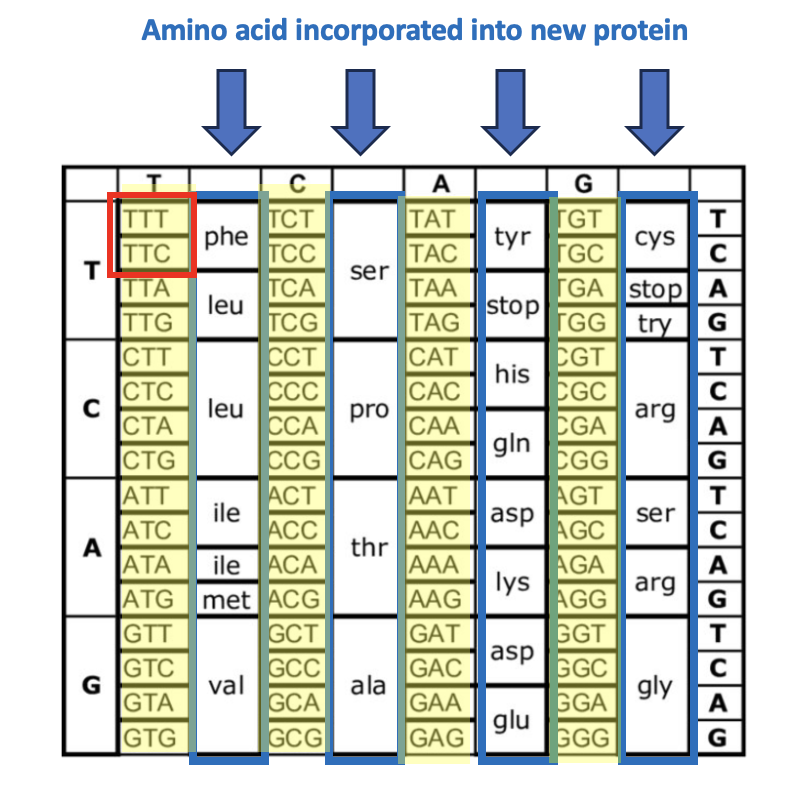
Figure 1. The genetic code (a set of instructions for DNA to make new proteins) determines which amino acids are incorporated into a growing protein and where they go. The blue arrows and boxes indicate the 20 amino acids that make up proteins. The mysterious-looking groups of three letters (yellow) are the instructions that tell DNA which amino acid goes next. For example, the red box shows two triplets, thymine-thymine-thymine (TTT) and thymine-thymine-cytosine (TTC). These are both sequences that are responsible for selecting the amino acid phenylalanine (Phe). Now, let's focus on the mutation responsible for SCD (Figure 2).
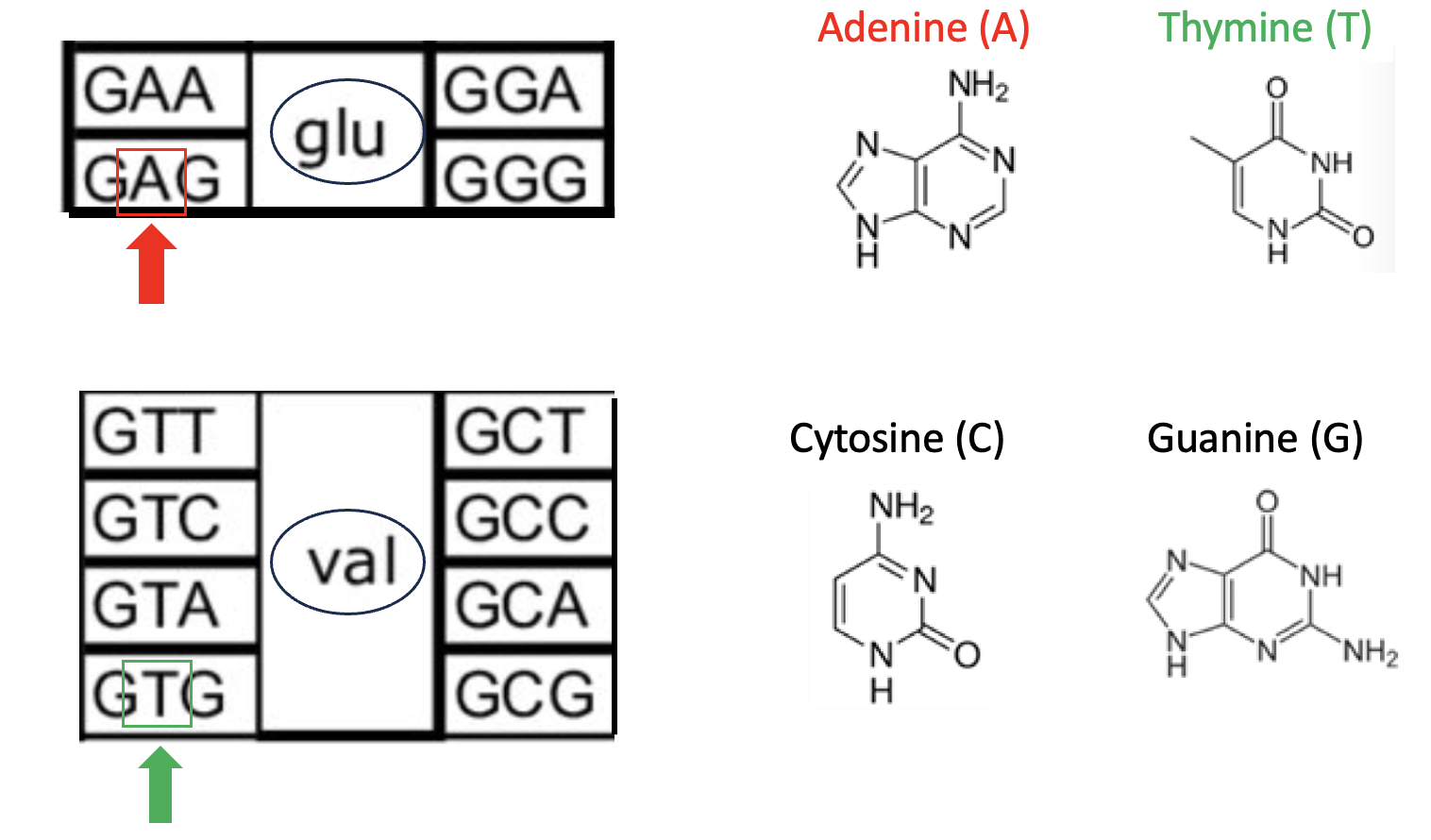
Figure 2. The glutamic acid to valine mutation.
The three-letter sequences of consecutive nucleobases are called a codon. The codon "grabs" the correct amino acid and "puts" it into the new protein. For example, when the enzyme that is building a protein – something I won't discuss here – encounters a DNA strand containing the codon (3 nucleobase sequence) GAA (guanine-adenine-adenine, top left), it knows to use (more commonly referred to as "codes for") glu – glutamic acid. Note that in that same box are three other sequences: GGA, GGG, and GAG. These codons also code for glutamic acid. (I won't go into the reason for this redundancy or the function of mRNA in the process.) This is the genetic code in its simplest form.
In the bottom box are 8 codons, any of which will code for valine. Why is this important? Because a comparison of the red box and green box shows a single-point mutation – the substitution of thymine for adenine. This seemingly trivial change – GAG to GTG means that people who have this mutation (both parents must have it) will end up with hemoglobin that contains Valine in the place where the glutamic acid normally goes.
Valine vs. glutamic acid: A small and big difference
When the single-point mutation has such a profound impact on hemoglobin function it must mean that there is an inherent difference between glutamic acid (no SCD) and Valine (SCD). This may seem crazy, but it is where "like dissolves like" comes into play (Figure 3).
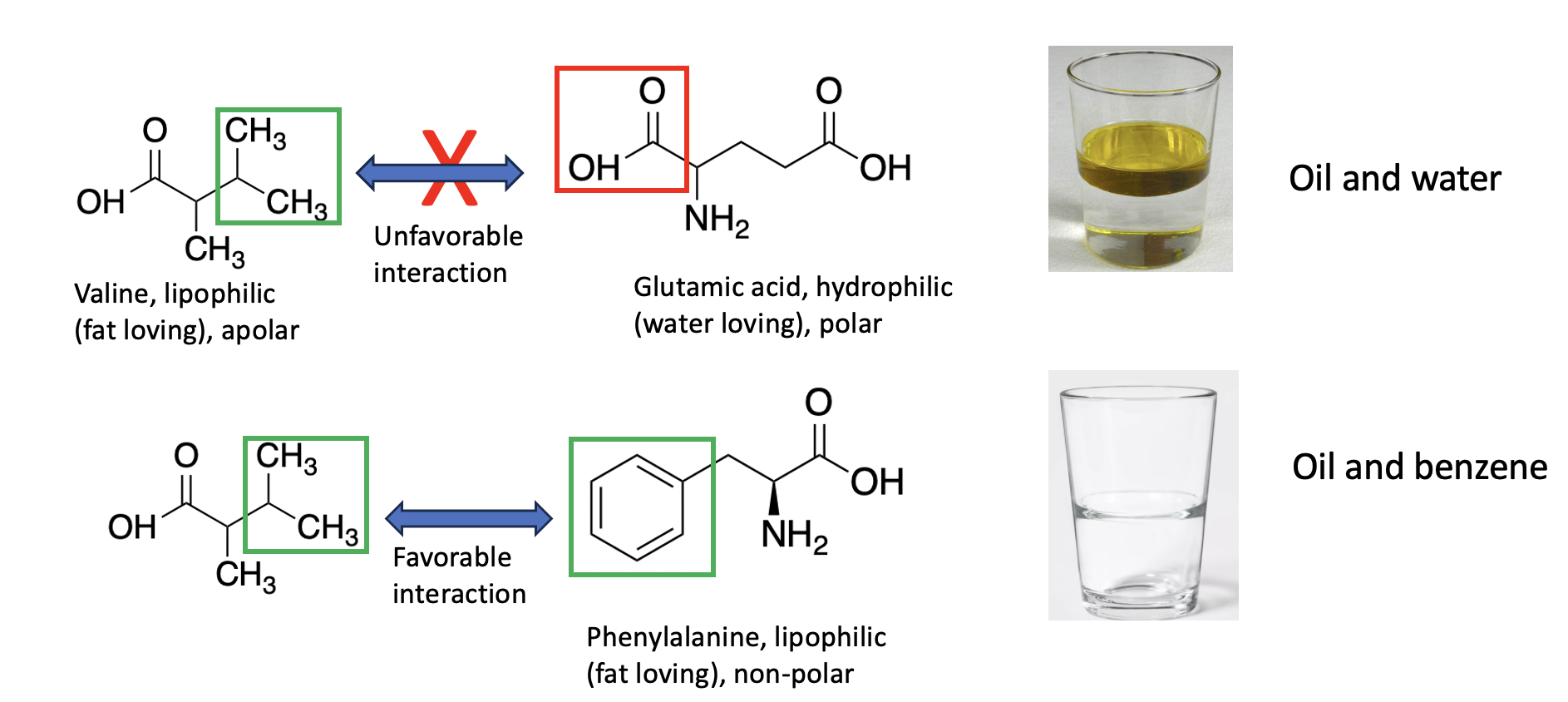
Figure 3. (top) Valine contains a three-carbon group with no oxygen or nitrogen atoms. This classifies it as a hydrophobic (lipophilic) amino acid. When an organic chemical contains carbon atoms with no polar groups, it is lipophilic (examples: gasoline, benzene, paint thinner, and oil) and it will be water-insoluble but fat-soluble. Like oil and water or vinegar, polar and non-polar substances will separate into two layers. Valine is more like oil than glutamic acid due to the polar carboxylic acid in the latter. The two will not be attracted to each other and may even be repelled. But valine and phenylalanine (bottom) both contain non-polar side chains and, just like oil and gasoline or benzene, they will be attracted to each other.
What does this have to do with SCD?
Plenty, and it starts to become evident in Figure 4.
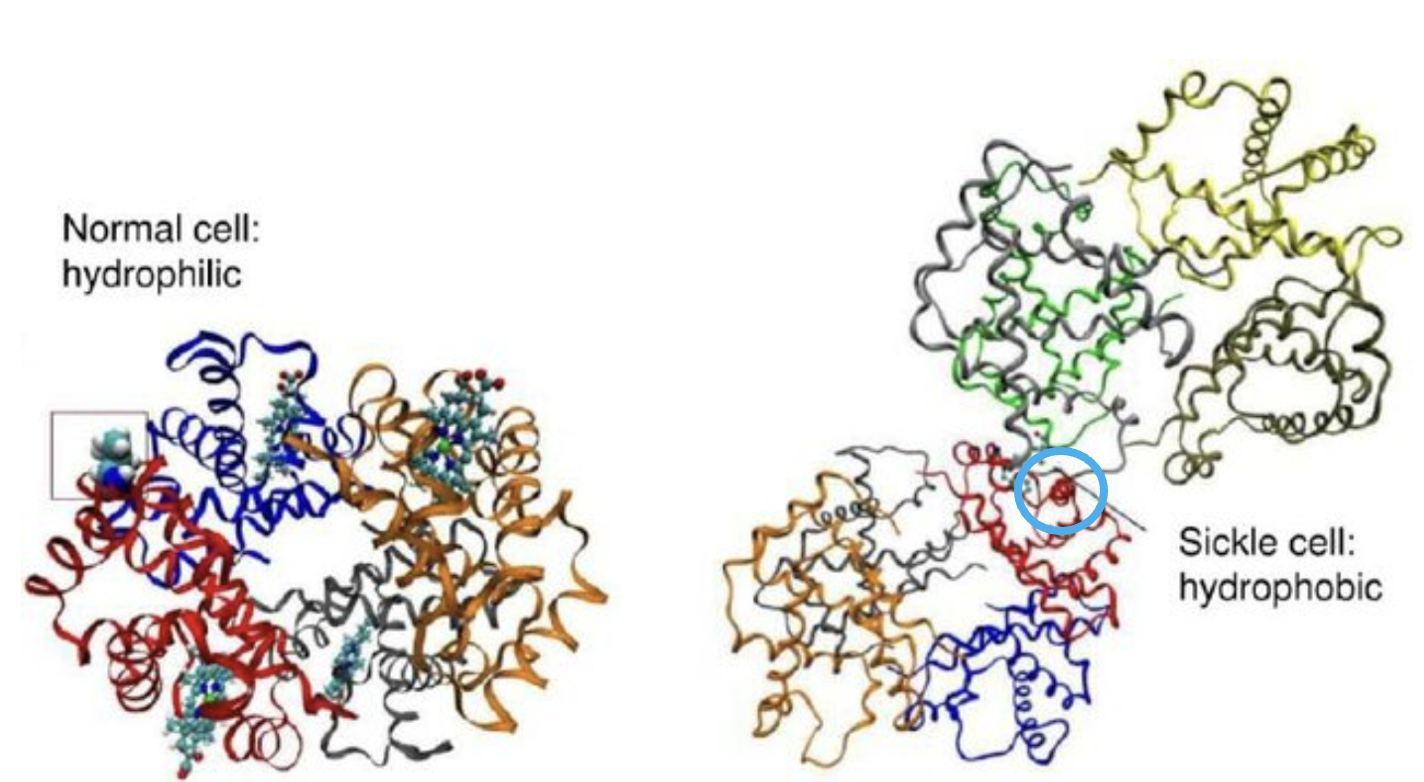
Figure 4. (Left) Normal hemoglobin. (Right) SCD hemoglobin. Note that in the SCD hemoglobin, two units are "stuck" together (light blue circle). This is the origin of SCD. Original image: Wu et al., Molecular Modeling of Normal and Sickle Hemoglobins, DOI: 10.1615/IntJMultCompEng.v8.i2.80
Why would a seemingly simple change in a large molecule make such a difference? It's as simple as oil and water (Figure 5).
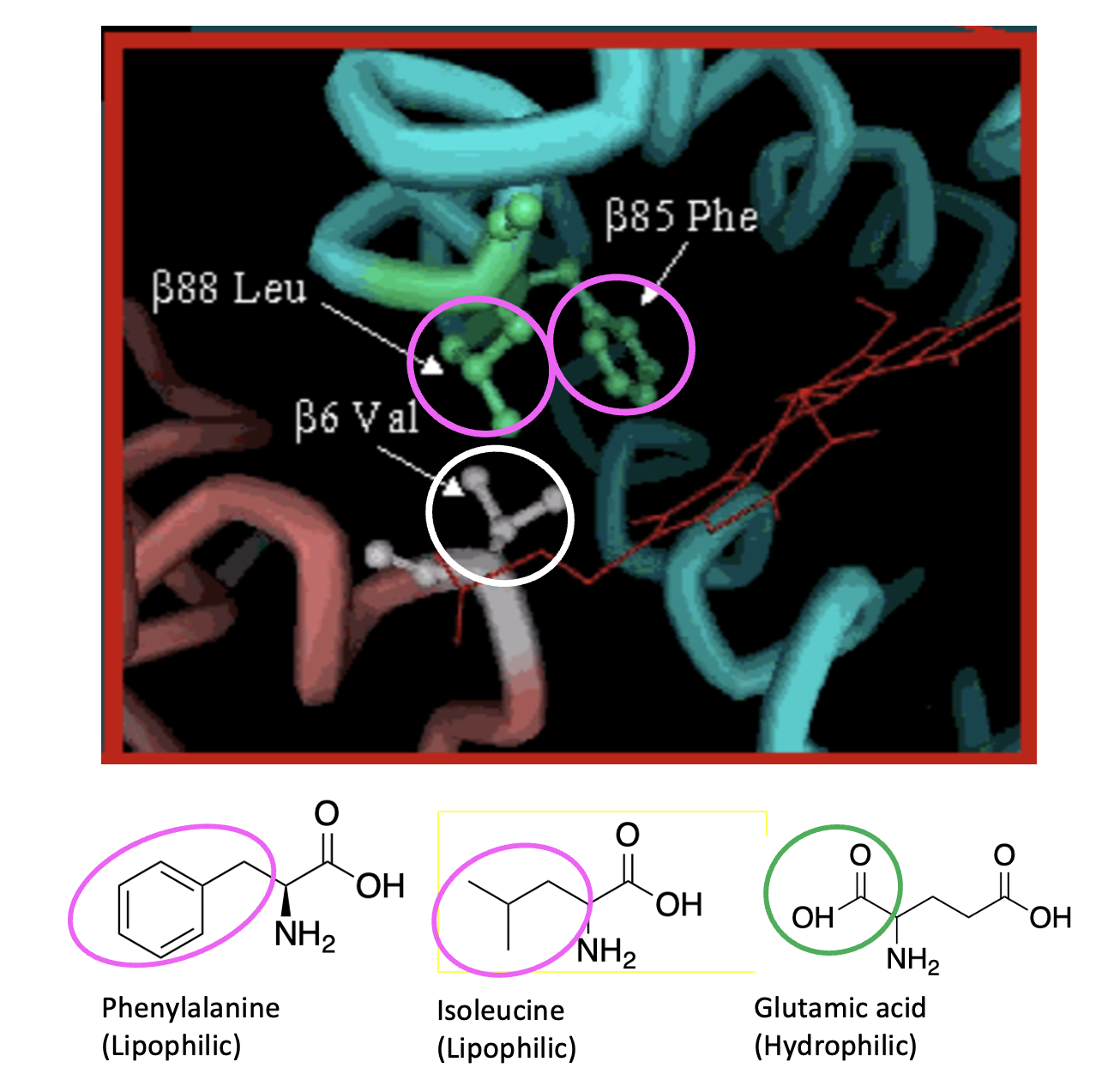
Figure 5. The impact of the glutamic acid valine mutation.
Valine (val) (white circle), being lipophilic, has a strong and favorable interaction with leucine (Leu) and phenylalanine (phe) (pink circles), which are part of a lipophilic fragment of another mutated hemoglobin molecule. This single interaction causes the proteins to clump together. Figure 6 (below) shows the effect when multiple hemoglobin molecules clump together and form long rods. This clumping profoundly affects the shape (and function) of the afflicted red blood cells.
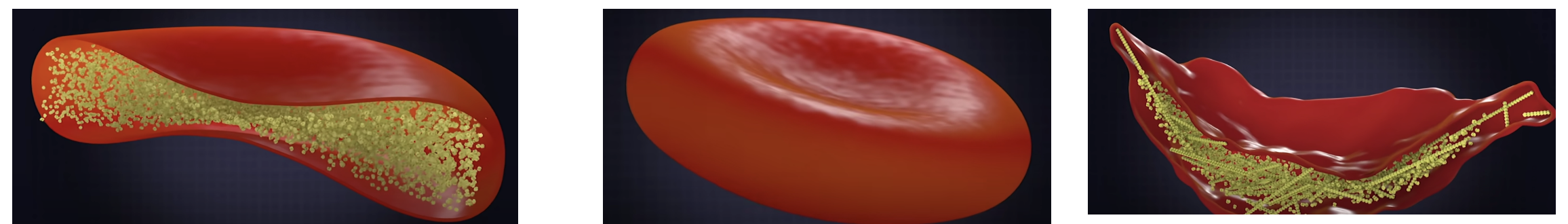
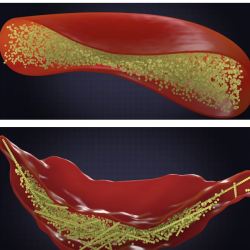
Figure 6. Normal hemoglobin (left, yellow particles) allows red blood cells to form a normal shape (middle). But when the glu ---> val mutation is present, the proteins form large inflexible structures that give sickle cells their characteristic shape (right). Source: Ted-ED video on YouTube created by Amber M. Yates.
Simple chemistry, tragic results.
It would be difficult to come up with a better example of how fundamental organic chemistry can profoundly impact human disease. The SCD mutation changes a water-soluble, hydrophilic group on a single amino acid to a fat-soluble lipophilic group. Oil and water don't mix. Unfortunately, valine does "mix" with chemically similar amino acids – just like droplets of oil attract each other on a water surface. The result is a horrible disease with a simple explanation.
NOTE:
(1) SCD affects approximately 100,000 Americans. SCD occurs among about 1 out of every 365 Black or African-American births. SCD occurs among about 1 out of every 16,300 Hispanic-American births.
