Almost all doctors and nurses in the US have been fully immunized. A survey asked them about getting a booster and the answer was an enthusiastic "yes." But doctors and nurses have somewhat different responses, probably due to a difference in risk tolerance.
Drugs & Pharmaceuticals
I've been following (and writing about) Antibe Therapeutics' otenaproxesul, an atypical NSAID without the GI toxicity of Aleve or Advil, since early 2020. It's been nothing but good news. Until now. Otenaproxesul caused a substantial jump in liver enzymes in some clinical trial participants. Can the company overcome this? Wall Street sure doesn't think so.
New data have been published on drug overdose deaths in 2020. Although you won't find it anywhere obvious, prescription opioid analgesics remain only a minor (and stable) contributor to the record 93,000 people who died from drug overdoses last year.
OK, the headline is a bit like clickbait, I do not believe Ivermectin is useful, but I could be wrong. (Did I just say that?) A new study demonstrates how a rush to publish, (and possibly treat) may have resulted in poorly designed studies where a quiet signal is lost in an abundance of noise.
The New York Times (correctly) reports that a COVID pill is needed, not just a vaccine. But the paper also tells this story in its typically biased manner, implying that the government, not drug companies, discover drugs. It's a bunch of nonsense that dates back to... forever.
Simple, inexpensive drugs to treat COVID are few and far between. But we may have a new, old pill to add to the arsenal. A new study tells us how well it works.
I've written numerous times that when it comes to supplements, you can throw both common sense and science out the window. Up is up and so is down. Somehow, I’ve been laboring under the notion that I don't really have much else to write about this topic. That was until a leisurely stroll up and down the aisles of a CVS store. And an existential thought experiment at no extra cost.
Drug companies try all kinds of nonsense to keep selling their brand-name drugs, when the same drugs are also sold as over-the-counter store-name generics. Sanofi just started a sleazy marketing campaign trying to do just that. And it's not their first time either.
Certain providers recently received a solicitation from Cigna, informing them that their patients on secukinumab (Cosentyx) – a biologic commonly prescribed for psoriasis, psoriatic arthritis, and other autoimmune illnesses – may soon receive a $500 debit card for accepting an alternative treatment. Uniformly, any patient on this medication is enticed to switch even if they’re stable on their current treatment. Disturbingly, this attempt to lure patients by providing them with a one-time payment blurs the line between insurance coverage and medical practice.
Are there methods that can be employed to ensure the commercial success of antibiotic biotech? ACSH advisor Dr. David Shlaes has his doubts.
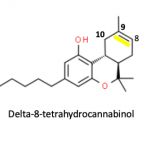
As if the confusion over marijuana, THC, and CBD oil isn't enough – both legally and pharmacologically – now there's a new wrinkle. It’s called Delta-8 THC, a previously minuscule and unimportant component of cannabis, which is virtually identical to THC itself. It's now possible to make the drug, also called "THC-light," from CBD oil. What does this mean? Read on. If you dare.
Another steroid has been found to prevent serious COVID, but this one is different. Unlike dexamethasone, which is a systemic steroid, budesonide, a drug commonly used for asthma, is dosed directly to the lungs, which makes it much safer. And it seems to work rather well.