The FDA is conducting a workshop to discuss the science (lack thereof, really) of Morphine Milligram Equivalents as it applies to the atrocious CDC 2016 Opioid Prescribing Guidelines. Public comments have been solicited. Here are mine.
Nearly three years ago I wrote how the science behind US opioid policies was deeply flawed, in particular, the use of Morphine Milligram Equivalents (MME) to quantify recommendations, policies, or laws. I argued that any use of MME was automatically flawed because it ignored even the most basic tenets of pharmacology, the absence of which made it impossible to rationally determine the relative strength of one drug to another. Unfortunately, this methodology, which became the foundation of the CDC’s catastrophic 2016 publication Prescribing Guideline for Prescribing Opioids, has metastasized ever since as one state after another has passed laws limiting the prescribing of opioid analgesic, often based on the Guideline's erroneous conclusions.
In retrospect, it is not surprising that such a baseless document was created. First, the CDC lacks both the authority and expertise to regulate drugs; that is the function of the FDA. Second, the anti-opioid group Physicians for Responsible Opioid Prescribing (PROP), which also lacks expertise in drugs and pharmacology, has undue influence with the CDC – a relationship that remains nebulous to this day.
It was almost a foregone conclusion that the CDC "recommendations" would become law. Indeed, this is now the case in 36 states. Sadly, drug abusers, pain patients, and their physicians have paid a very steep price for this ill-conceived document.
Although it is years too late, it is nonetheless encouraging that the FDA is holding a workshop to examine the fundamental pharmacology of opioids. The deficiencies of the 2016 Guide need to be addressed. Here are my comments.
Can Opioid Drugs Be Directly Compared?
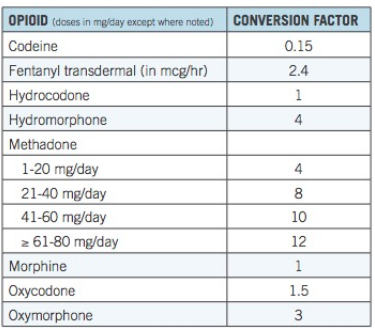
Table 1. Opioid conversion factors. Source: CDC
Table 1 forms the basis of the MME approach. Its basic premise is that different opioid drugs can be quantitatively compared and the relative strength of each opioid drug used to establish dosing limits for individual drugs. For example, if 90 mg of morphine (90 MME) is set as the upper limit for a daily dose, then the maximum allowable doses for oxycodone and oxymorphone would be 60 mg and 30 mg, respectively. But if the assumptions in the table are incorrect then any use of them to form policy must also be incorrect.
The primary reason that the MME conversion table is inadequate is that it fails to take simple pharmacological principles into account. In order to understand this limitation, we need to examine some of the fundamental principles of pharmacokinetics – the way that the body handles a drug (2). Arguably, the most important parameter is metabolism. It is critical in determining the fate of a drug once it enters the blood and by extension, the proper dose.
Metabolism
The purpose of metabolism, which occurs almost exclusively in the liver, is to break down and eliminate drugs and other chemicals from the blood – the body's "detox" system. Metabolism can be subdivided into two distinct phases, based on the type of chemical transformation and the enzymes that are responsible for this transformation.
Phase 1 enzymes are members of the Cytochrome P450 enzymes (CYP family). The function of CYP enzymes is to break down drugs. In contrast, Phase 2 enzymes are not members of the CYP family; they have unique names, such as UGT and SULT, abbreviations for the name of enzyme carrying out a specific reaction. Phase 2 enzymes don't break down molecules; they add to them. Although they act in different ways, the primary function of both enzyme classes is to facilitate the elimination of xenobiotics.
Phase 1 vs Phase 2 Metabolism
The difference between Phase 1 and Phase 2 metabolism can be illustrated by examining the metabolic fate of two simple, naturally occurring chemicals. Anisole, (aka methoxybenzene) is one of the essential oils that give licorice its flavor and scent. Phenol (aka hydroxybenzene) has antiseptic and anesthetic properties. Although they are similar in structure – differing only by one carbon atom – the two chemicals are metabolized very differently.
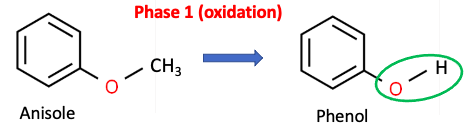
Figure 1. Phase 1 enzymes such as CYP2D6 and CYP3A4 catalyze the oxidation of anisole to phenol. As is common in Phase 1 reactions, a hydroxyl group (green oval) is formed.
Upon consumption, anisole is converted by cytochrome P450 (CYP) enzymes to phenol (3) as shown in Figure 1. Note the loss of the methyl group, leaving a hydroxyl group (green oval) in its place. As is the case with anisole, Phase 1 metabolism is (normally) an oxidation reaction, in which lipophilic (fat-loving, poorly water-soluble) molecules, are broken into smaller fragments that are more hydrophilic (water-loving and water-soluble). Phase 1 metabolites often contain hydroxyl (OH) or amino (NH2) groups.
Phase 2 metabolism is fundamentally different. Phase 2 enzymes conjugate or add water-solubilizing groups to a drug or its metabolite to facilitate the elimination of the drug in the urine. One example is the sulfation of phenol shown in Figure 2.
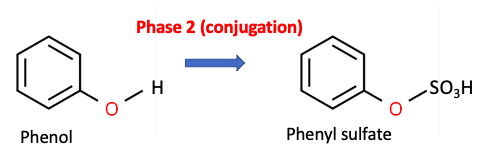
Figure 2. Phase 2 metabolism - The PST enzyme catalyzes the conversion of phenol to phenyl sulfate (aka phenol hydrogen sulfate), which is then excreted in the urine.
The hydroxyl group in phenol acts as a chemical "handle" that allows the attachment of a water-solubilizing group, such as sulfate and this plays a critical role in elimination. (The abbreviated name of the enzyme that promotes this reaction is PST, short for Phenol SulfoTransferase.)
What Does This Have To Do With Opioid Dosing?
Plenty. This can be seen by examining the structures of oxycodone (Figure 3, Left) and oxymorphone (Right). and their similarity in structure to anisole and phenol, respectively.
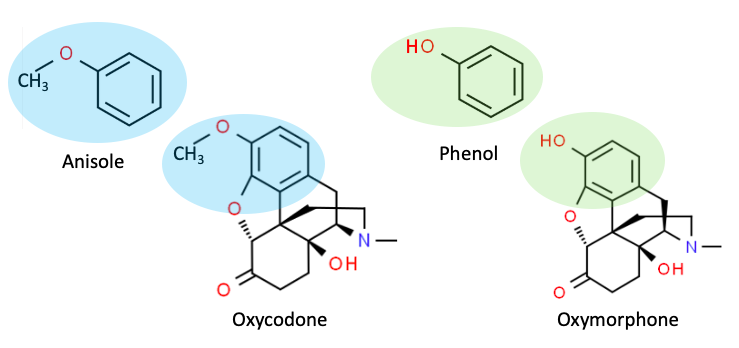
Figure 3. Anisole and oxycodone both contain methoxy groups, which makes them substrates for Phase 1 enzymes. Phenol and oxymorphone both contain hydroxyl groups, which makes them substrates for Phase 2 enzymes.
As shown in Figure 3, anisole and oxycodone are structurally similar in that they both contain a benzene ring bearing a methoxy group (shaded blue). This makes them substrates for Phase 1 CYP enzymes (oxidation). By contrast, phenol and oxymorphone are similar in that they both belong to the phenol class – a benzene ring bearing a hydroxyl group (shaded green). The phenyl hydroxyl (phenol) group makes both of these chemicals substrates for Phase 2 enzymes (conjugation). The second row in Table 2 shows the primary metabolic enzyme(s) for each drug.
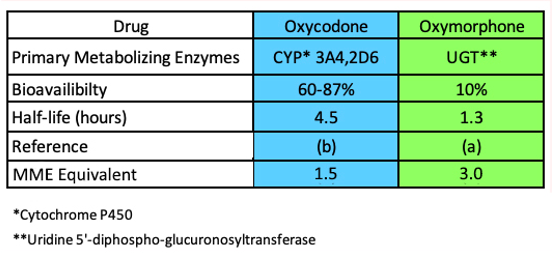
Table 2. Three pharmacokinetic parameters of oxymorphone (right) and oxycodone (left). Source: Ref. (a). Ref. (b).
Direct Comparison of Opioid Drugs – the Essence of MME – is Scientifically Unsound

The two opioid drugs at the bottom of Table 1 are metabolized by a different family of enzymes and at a different rate, yet all the CDC table tells us is that one is twice as strong as the other. It's quite tidy to state that oxycodone is 1.5-times stronger than morphine and that oxymorphone is twice that, but what does this mean? Perhaps this is true in a binding assay or even clinically in some people but the statement is, at best, oversimplified. But the metabolism of each drug is radically different, which affects the amount of drug present in the blood and brain.
Metabolism Isn't the Only Difference: Bioavailability and Half-life
Table 2 shows two other pharmacological parameters, bioavailability, and half-life, which are equally important in determining the properties of drugs.
Bioavailability is a measure of how efficiently an orally administered drug gets absorbed into the blood. The higher the number the higher the absorption and resulting blood concentrations. Table 2, row 3 shows that the bioavailability of oxycodone (60-87%) is significantly better than that of oxymorphone (10%).
Half-life (Table 3, row 4) is the amount of time that it takes for the concentration of the drug in the blood to decrease by 50%, a function of metabolism. The half-life of oxycodone (4.5 hours) is 3-4 times that of oxymorphone (1.3 hours), so it stays in the blood much longer.
So, which is a better pill?
The CDC chart says that oxymorphone is three times as strong as morphine, but much less of it gets into the blood and when it does, it is metabolized and excreted quickly. Oxycodone is supposedly twice the strength of morphine, but is well absorbed and remains in the blood for considerably longer. Which is a better pill? The answer is: who knows? But it sure isn't as simple as what the CDC guideline state – that 300 mg of morphine is equivalent to 100 mg of oxymorphone counts and 150 mg of oxycodone. Convenient? Yes. Accurate? Absolutely not.
Bottom Line
It is incomprehensible that the CDC would put out a series of guidelines without bothering to consider even the most fundamental tenets of pharmacology. But this is what happens when a woefully uninformed group like PROP feeds advice to an equally clueless CDC and lawmakers pick up the baton – a shameful and disastrous chapter in American medical history, which has resulted in millions of un- or undertreated pain patients in addition to a stark increase in overdose deaths as fentanyl has "filled the gap" created by the tightening of prescription of analgesic opioid drugs.
The failure to consider even these simple metabolic differences is a primary reason why the CDC table fails as a useful guide and why the concept of morphine milligram equivalents is scientifically faulty. Poor science will necessarily lead to poor policy. We have enough of both.
NOTES
(1) There is a significant genetic variation in the levels of metabolic enzymes from one individual to another. This is beyond the scope of this document.
(2) Pharmacokinetics is a subset in the study of pharmacology that focuses on the effect of the body on the drug. Common pharmacokinetic parameters include absorption, bioavailability, distribution, metabolism, and excretion.
(3) It is unusual for a molecule to be metabolized to a single metabolite. Anisole, phenol, oxycodone, and oxymorphone form multiple metabolites. I have intentionally omitted these. They are not relevant to the discussion and would add nothing to the article but confusion.