It's intuitive that a robust immune response to COVID-19 will result in a less severe, even asymptomatic infection. A new study puts some numbers to the term "robust."
The study, in 92 critically ill patients with PCR positive COVID-19, looked at the presence of IgG and IgM antibodies directed specifically at COVID-19 as well as the presence of viral RNA and COVID-19 specific proteins.
- IgM is the SWAT team of antibodies first on the scene when a foreign antigen is detected.
- IgG is IgM’s replacement, with levels risings as those of IgM fall. They are responsible for long-term protection creating a “catalog of IgG antibodies that can be rapidly reproduced whenever exposed to the same antigen.”
- Viral RNA and those COVID-19 specific antigens, the presence of which is termed antigenemia, reflect the expression or presence of COVID-19 – viral load can be used as a comparable term
The researchers collected their samples within 24-hours of a patient’s admission to the ICU. They compared the 30-day survivors to the 41% (38) that died in the same period. As you might expect, those dying were older (70 vs. 60) and more frequently had high blood pressure and diabetes, among the considered co-morbidities. Their severity scores [1] were also higher.
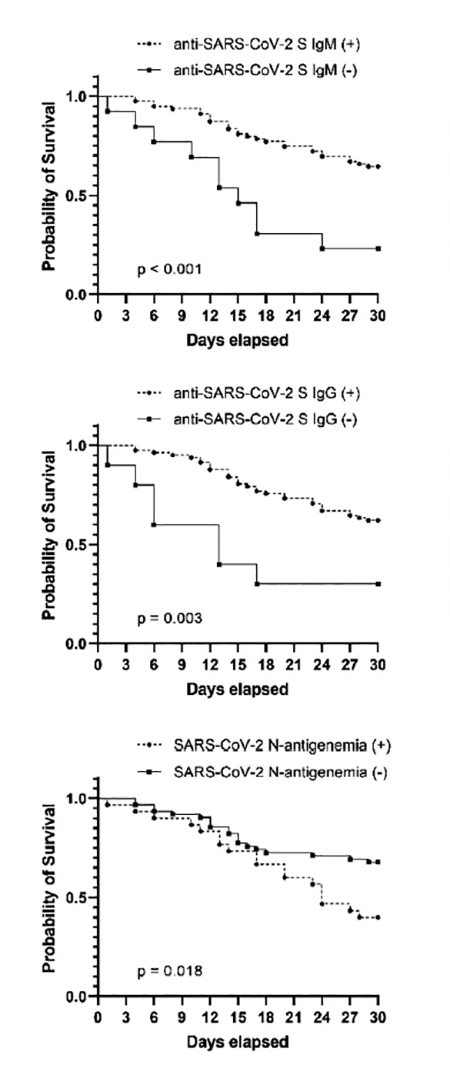 Those patients with IgG and IgM had a more “muted” clinical course; it took 8 to 10 days from the onset of symptoms to requiring ICU admission. For those with low or no levels, ICU admission occurred within five days.
Those patients with IgG and IgM had a more “muted” clinical course; it took 8 to 10 days from the onset of symptoms to requiring ICU admission. For those with low or no levels, ICU admission occurred within five days.- Patients who died had lower or absent levels of IgG and IgM and higher levels of viral RNA and COVID-19 antigens.
- Both the immune response and the viral load were independent measures of survival.
On the right are the survival curves based upon the presence or absence of IgG, IgM, and viral “antigenemia.”
In their discussion, the researchers provide a few insights that may be of value to our understanding of this infection. Clearly, a robust response by our immune system is necessary to survive. It appears that these antibodies dampen the spread of the virus within us. The reduction in the presence of viral antigens, in turn, may reduce the possibility of a diffuse, uncontrolled, inflammatory response – cytokine storm. Medications and hospitalization are necessary supportive measures.
For patients whose vital systems, hearts, lungs, kidneys are compromised by co-morbidities, medications and hospitalization are necessary to support bodily function while an intact immune system has a chance to work. The researchers identify “cutoff” levels for the presence of viral RNA and protein, IgG, and IgM associated with mortality. For those whose immune response is weakened, and we can use these four biomarkers as the signs of weakness, support need also be directed at our immune response.
The authors speculate about the metric's utility in identifying those who need a prescription of exogenous antibodies - convalescent serum. Convalescent plasma has had mixed results in treating COVID-19. The most recent study found it had no efficacy in those outpatients with early symptoms, but it has helped some critically ill patients, but not all. We could be treating the wrong patients because our stratification, based on indirect symptoms and signs, i.e., the need for ventilation or dropping oxygenation, doesn’t reflect the immune response. The value of today’s research is that it provides a metric of immune response that may stratify critically ill patients in a manner where convalescent plasm is more effective.
[1] Critical care patients are often stratified using standard severity scores because different bodily systems can be critically ill. APACHE is one of the scoring systems used in this study; the acronym stands for Acute Physiology and Chronic Health Evaluation. You can read more about them here.
Source: Low anti-SARS-CoV-2 S antibody levels predict increased mortality and dissemination of viral components in the blood of critical COVID-19 patients Journal of Internal Medicine DOI: 10.1111/joim.13386