The EPA’s Integrated Risk Information System (IRIS) was founded in 1985 to assure scientific integrity and present intra-Agency consensus on health effects information on environmental chemicals. It is an excellent example of how a government agency can misuse a well-intentioned idea to meet a political agenda resulting in flawed public policy decisions with significant implications.
This article examines the IRIS assessment for ethylene oxide, showing how scientific inaccuracy can lead to flawed public policy decisions with significant implications.
What is IRIS?
IRIS was established as a centralized location to present EPA’s risk assessment conclusions on environmental chemicals. IRIS assessments consist of qualitative summaries of available health effects data and quantitative values calculated to be “safe” levels for the chemicals in the environment. IRIS assessments are not based on any legal or statutory authority but are often used by EPA offices and States as the basis for regulations and private companies to assess risk.
There has been controversy over EPA’s claims that the information in IRIS is impartial, accurate scientific information that objectively measures the risk of chemicals. Issues include IRIS’ scientific inaccuracy, lack of transparency, limited peer review, and slow progress in producing assessments. These issues and others have been examined in congressional hearings, Government Accountability Office reports, and an Inspector General report. Although much talk has been about reforming IRIS over the years, little meaningful action has been taken.
Ethylene Oxide Illustrates the Problem
Ethylene oxide is a chemical intermediate in producing many consumer products, including detergents, adhesives, plastics, textiles, antifreeze, and personal care products. The human body naturally has appreciable levels of ethylene oxide, and it also is produced by vegetation, waterlogged soil, manure, fires, vehicle emissions, and smoking. Arguably, its most important use is to sterilize medical devices, sterilizing over 20 billion medical devices yearly.
In 2016, EPA produced a revised IRIS assessment for ethylene oxide, focusing on its cancer hazard when inhaled, replacing earlier reviews from 1998 and 2006. The 2016 assessment changed EPA’s cancer risk descriptor for ethylene oxide from “probably carcinogenic to humans” to “carcinogenic to humans.”
To reach that conclusion, the EPA used both animal and human studies. Animal studies showed an increase in various tumor types after mice were exposed to high levels of ethylene oxide for two years. The results from three National Institute of Occupational Safety and Health (NIOSH) studies [1] tracking 18,254 workers at 14 facilities exposed between 1938 and 1985 to ethylene oxide used to sterilize medical equipment failed to find a statistically significant, causal relationship between ethylene oxide and cancer. In fact, these studies showed that cancer rates were lower in the workers exposed to ethylene oxide over several decades than in the general population.
Based on inconclusive results from some of the workers in these studies, the EPA concluded that ethylene oxide caused an increase in breast cancer and hematopoietic (blood) cancers. A systematic review and meta-analysis of 30 studies published between the 2000s and 2010s did “not support the conclusion that exposure to ethylene oxide is associated with an increased risk of blood cancers or breast cancer” but was not included in the EPA’s assessment.
The EPA used the Supra-Linear model and the NIOSH studies data to calculate ethylene oxide's cancer risk. The EPA usually uses the Linear Non-Threshold mode for dose-response assessment. It assumes a direct and proportional relationship between dose and cancer risk over the full range of doses. The Supra-Linear model increases the proportional risk at lower doses resulting in lower doses carrying more risk than the Linear Non-Threshold model would suggest.
EPA calculated that exposure to ethylene oxide at a concentration of 0.1 parts per trillion (ppt), equal to 0.0001 parts per billion (ppb) in the air, presents a one-in-a-million lifetime cancer risk (the level that government agencies often use to represent a significant cancer risk and to regulate a chemical). THE EPA's use of this model has been questioned as it significantly over-predicts risk and is infrequently used in risk assessment.
The Texas Commission of Environmental Quality (TCEQ), dissatisfied with the EPA calculation, recalculated the ethylene oxide’s cancer risk using the same data but the Cox Proportional Hazards model, a more widely accepted model. This resulted in an estimate of cancer risk that was 4,000 times lower than EPA’s estimate.
The Texas model more accurately predicts cancer risk.
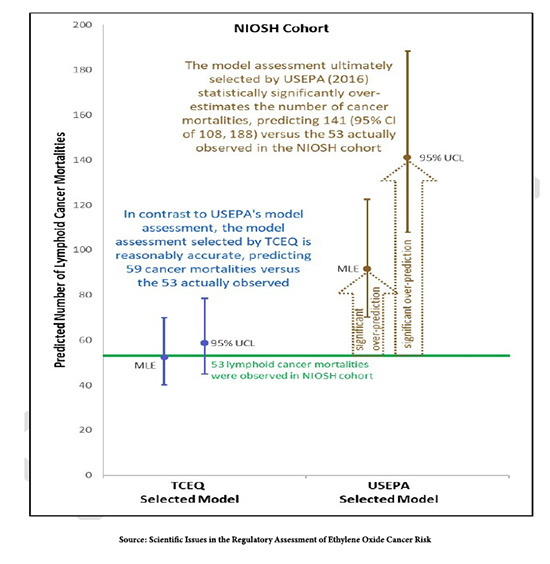 The graph shows that the NIOSH studies' data fitted to both models. The NIOSH studies showed 53 deaths from lymphoid cancer after exposure to ethylene oxide in workers. The model used by Texas predicted 59 deaths, very close to 53. The model used by the EPA predicted 141 deaths, two and a half times greater than the actual number of deaths.
The graph shows that the NIOSH studies' data fitted to both models. The NIOSH studies showed 53 deaths from lymphoid cancer after exposure to ethylene oxide in workers. The model used by Texas predicted 59 deaths, very close to 53. The model used by the EPA predicted 141 deaths, two and a half times greater than the actual number of deaths.
The EPA has not acknowledged these results and has continued to defend its model and assessment even when it is not supported by real-world data.
The human body naturally produces 0.56-4.5 ppb of ethylene oxide; typical urban concentrations are approximately 0.1-0.2 ppb. Based upon the EPA’s assessment, Ethylene oxide causes a significant cancer risk at a level 19,000 times below the level found naturally in the human body and 1,000 to 2,000 times below that found in typical urban air.
Sterilizing Medical Equipment
EPA uses the IRIS assessments to determine risks in regulatory and non-regulatory actions that tremendously impact the public.
The EPA recently proposed reductions in the amount of ethylene oxide plants can use to sterilize medical equipment. This rule could have substantial negative repercussions across the U.S. According to the CEO of the Advanced Medical Technology Association (an industry lobby group), “stricter regulations could force sterilization facilities to close, leading to treatment delays as sterile technology supplies, such as pacemakers and surgical equipment, fall short.” This rule involves significant costs based on greatly overestimated cancer risks.
The EPA’s National Air Toxics Assessment (NATA) provides community screening assessments. Writing in their 2018 fact sheet
“The 2014 NATA estimates that ethylene oxide significantly contributes to potential elevated cancer risks in some census tracts across the U.S. (less than 1 percent of the total number of tracts). These elevated risks are largely driven by an EPA risk value that was updated in late 2016.”
This caused increased anxiety and stress in some communities near medical equipment sterilization plants, with some plants shut down in 2019 due to fear of cancer risks.
What to do About IRIS?
The problems with IRIS are not unique to ethylene oxide. As I have written, the IRIS assessment that chromium-VI is likely to cause cancer through drinking water is not supported by the scientific data, which could result in drinking water regulations with significant costs and little public health benefit.
IRIS should be reformed by clarifying that the individual EPA Offices with statutory authority over a chemical have primary responsibility. For example, ethylene oxide is regulated under the Toxic Substances Control Act (TSCA), so the Office of Pollution Prevention and Toxics should prepare the IRIS assessment. Bring the IRIS process under the Administrative Procedure Act (APA) that dictates how federal agencies develop rules and regulations. This would bring greater transparency to the basis for IRIS determinations.
[1] Mortality among workers exposed to ethylene oxide, Mortality analyses in a cohort of 18 235 ethylene oxide exposed workers: follow up extended from 1987 to 1998, and Ethylene oxide and breast cancer incidence in a cohort study of 7576 women (United States)