Once again the press (and a whole bunch of scientists) got it wrong. Halfway anyhow.
After years of debate, the FDA finally made the decision to ban antibacterial hand soaps. Good for them. The soaps are worthless, and possibly harmful, something I wrote about in 2015.
The decision makes perfect sense, especially if you consider the risks and benefits of triclosan, the bacteria-killing chemical which is now banned from hand soap. Triclosan containing soaps did nothing more to prevent disease than washing your hands with plain old soap. If the benefit of adding a chemical is zero, then the risk to benefit ratio is automatically infinity, even if the chemical in question has even a small risk.
Triclosan is potentially harmful, but not for the reasons given in the press. You've seen much of the same: unfounded (or nonexistent) fears that this or that was messing up your hormones. Just another example of the ubiquitous (and quite stale) endocrine disruptor blather, which is a standard practice used by know-nothing organizations like the Environmental Working Group, a group that is wrong on just about everything. It is a superb way to generate fear among the public in order to get chemicals banned.
The essence of phony endocrine disruptor scares is two-fold:
- The chemical of choice is shown to affect a receptor, usually an estrogen receptor. However, the degree to which the chemical in question binds to the receptor (the relative binding affinity,) compared to the natural substance, (in this case, estradiol) is rarely, if ever, discussed.
- The relative blood levels of the chemical and estradiol are rarely given.
In the absence of this information, people are left with the impression (which is purely intentional) that all of these "awful" chemicals are getting into our bodies in huge amounts, where they elicit a profound physiological effect by screwing up the regulatory system of hormone-receptor interaction and function.
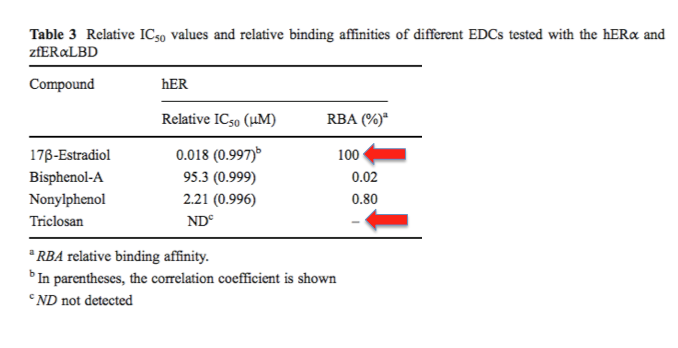
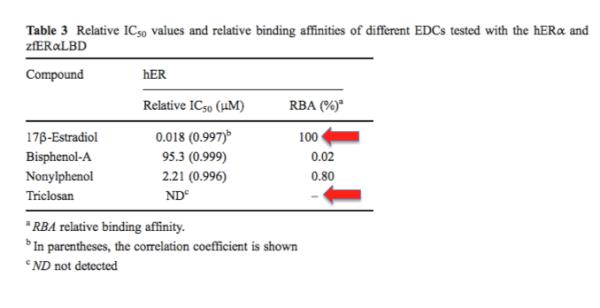
Almost without exception, it's a bunch of garbage. Below is one example (out of hundreds) of what happens when these parameters are actually measured (1).
(source: ResearchGate)
In the above table, three chemicals are compared to 17-ß-estradiol (one of the endogenous sex hormones) in an assay that measures relative binding affinities. On the left, 17-ß-estradiol's binding affinity for a human estrogen receptor is compared to that of three common chemicals: triclosan plus two other common substances. One of them is the "dreaded" BPA. In this assay, BPA binds 5,000-times more weakly than estradiol. But at least it can be measured. Triclosan has such a weak affinity for the receptor that no binding could even be measured so the chances of it affecting your sexual development (or anything else) are approximately zero.
None of these facts stop the NRDC from making one more of their seemingly-endless endocrine disruptor screeds: "Dangerous Chemical in Soaps and Toothpaste Facing Closer Scrutiny." It is very easy to call something an endocrine disruptor when you fail to mention that it would only be possible, if at all, at such a high concentration as to make the assay completely irrelevant to human health.
So, here are the three legitimate reasons to get rid of triclosan in hand soap:
1. It doesn't work, which is not particularly surprising, especially when you consider that the most commonly transmitted infections—colds, influenza, and norovirus (aka "the stomach flu") are viruses, which are going to sit there laughing at a chemical that was designed to kill bacteria.
2. Adding triclosan wastes the raw materials needed to make it and the energy required to transport it.
3. Pay attention to this one. The real harm of using triclosan is not disruption of hormones; it is the potential of promotion of bacterial resistance to antibiotics. And, there is no one better at explaining this than Dr. David Shlaes, a premier expert in the fields of antibiotics and microbiology, and one of the American Council's scientific advisors. Dr. Shlaes explains (emphasis mine):
"Bacteria can become resistant to triclosan by pumping the drug out of the bacterial cell before it can do any damage. These pumps probably exist to rid the bacteria of waste products and toxins that they either produce or encounter in the environment. Under certain circumstances, they can press the accelerator and these pumps work harder and faster as in the case of triclosan resistance. The problem is that these pumps are not very specific, and they can pump out a number of antibiotics as well as triclosan. In this way, triclosan resistance is actually resistance to multiple antibiotics. Not such a good thing."
It took too long, but the FDA finally banned a chemical that never should have been in hand soap in the first place. But, the press took the easy way out and gave credence to scientifically challenged chemical-hate groups, some of which have neither a chemist nor the slightest idea of how chemistry works.
We do. If you want the real answers to complex scientific questions, give us a try instead.
Note:
(1) This paper is long and complex, and is being used solely as an example of a large (in this case infinite) difference in receptor binding between endogenous hormones and hormone-like chemicals, not an example of poor science. I am not passing judgment on the quality of the work, or paper,