OK, this article is going to start off sounding boring, perhaps painfully so. If not for the three Dexies I just popped I doubt I'd even be able to finish the wretched thing. But, please bear with me. There is some very cool stuff coming up, but I'm not gonna share the Dexies. You're on your own.
It is remarkable what organic chemistry - the chemistry carbon-containing molecules (1) - can do. No one reading this that doesn't benefit from "orgo" in some way. It is responsible for virtually all drugs, your toothbrush, perfume and other consumer products, clothing, plastic and rubber, plumbing, cleaners, and Velcro. But organic synthesis - the practice of transforming existing molecules into new ones - also has a dark side. The fentanyl that is killing thousands of people each year is made by using the exact same techniques that any organic chemist would use when working in the lab. Fentanyl can be made by chemically combining and modifying four small fragments of the molecule, and it's very easy to do. Same with most street drugs.
Organic chemistry can also be used as a detection tool. For example, the Breathalyzer test is based on a simple 70-year-old reaction called the Jones oxidation. In the presence of alcohol, Jones Reagent (2) changes color from orange to green.
There has been much research regarding the detection of other drugs, mostly in urine. A number of recent reports describe rapid and easy tests which can detect amphetamines, which can impair driving performance (or turn you into a New Yorker, pretty much the same thing). A recent paper in the journal, Chem describes an electrochemical sensor system that does just this. Except I wouldn't understand it if I spent the rest of my life staring at the damn thing. It looks like this:
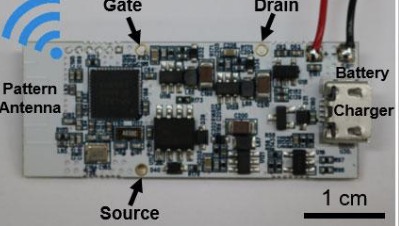
Point-of-Use Detection of Amphetamine-Type Stimulants with Host-Molecule-Functionalized Organic Transistors. If you can understand this, you can have my job. Source: Chem
There are plenty of other methods for detection of amphetamines which I don't understand either, including both gold and silicon dioxide nanoparticles. But there is a simpler method, which I will describe below, which uses a known chemical reaction and a plainly visible color change to detect meth in urine. Pretty cool stuff. If you keep going, you'll learn about this and also a little about why different chemicals have different colors.
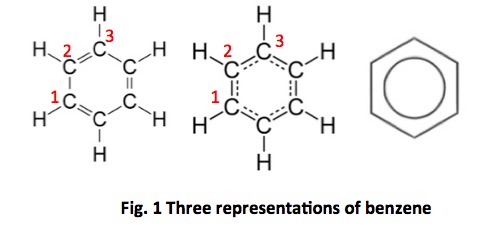
In organic chemistry, the most common type of bond (by far) is one that connects two carbon atoms; these can be single bonds or double bonds (triple bonds are far less common). Little known fact: When single bonds alternate with double bonds (this is called conjugation) they behave a little strangely; each bond has characteristics of both single and double bonds (Figure 1). In fact, as shown in the middle structure, the electrons of the double bond of the representation on the left are shared equally between the carbon atoms labeled 1, 2, and 3, which is more accurately shown in the middle figure. The figure on the right is shorthand for either of the others. This is why the structure of benzene is displayed in three different ways, all correct.
Conjugated bonds give organic molecules special properties. One is stability. Think of a chain where the links are all 1.5 mm in width compared to another chain with alternating 1 and 2 mm width links. Which will be stronger? Another property that conjugated bonds give molecules is color, at least when there are enough of them. You usually need about 8 in a row to start seeing colors.
Here's an example. Figure 2 shows two molecules, both consisting of long chains of carbon (ignore the CH3 (methyl) groups. They make no difference). The molecule on top is a called lycopene, the deep red pigment of tomatoes, and it has 11 consecutive conjugated bonds. Compare this to a long chain of carbon atoms connected that only by single bonds. This is a colorless wax.
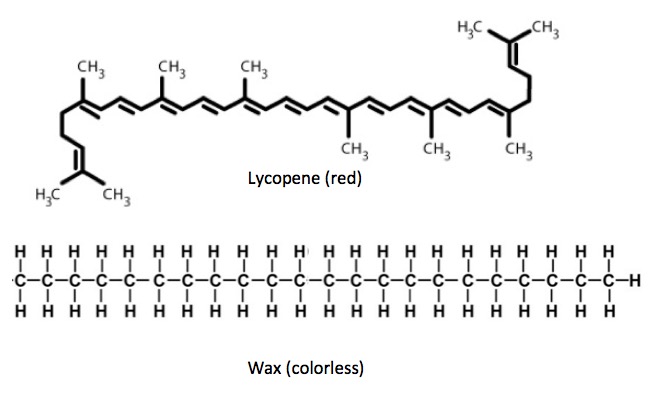
Figure 2 - The color of conjugated vs. non-conjugated compounds
This same concept has been applied to the detection methamphetamine (speed) in urine. (Figure 3). It involves the use of one simple chemical reaction. The blue compound (top) is one member of a class of colored organic molecules called azo dyes. (3) I have colored the double bonds of the single-double conjugated units with red circles. The blue dye contains a total of 14 of these units.
But the carbon bound to oxygen (blue circle) is called a ketone (4) and is highly reactive. It reacts immediately with the nitrogen atom of the amine group (NH2) on methamphetamine. The product of this reaction (bottom) now contains only 13 conjugated units. This is sufficient (5) to cause the blue dye to be converted into a red dye. (The green arrow indicates where the reaction took place. Note that there is no longer a double bond at this position.)
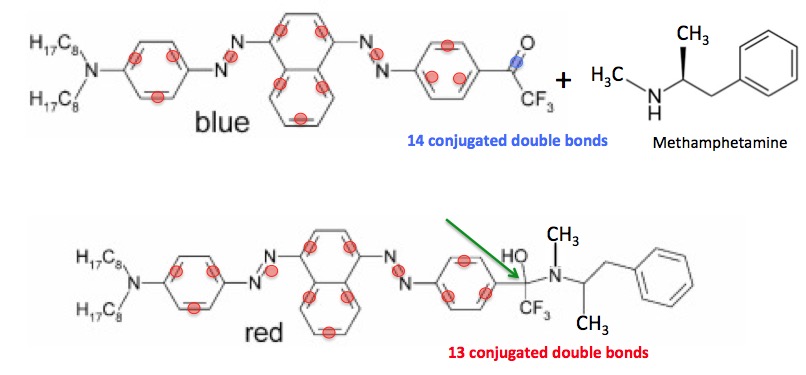
Figure 3 - The change in color caused by a chemical reaction that decreases the number of conjugated units.
So, it's all rather simple. Affix the blue dye to a chip, apply urine, look for the color change, and bingo - if it's red, you've been caught red-handed. You'd better not "speed" while driving. Organic chemistry, plus whatever your insurance company may do, will leave you in the red.
Question: There is one possible problem with this method - false positives. Any guesses?
(1) Virtually all molecules that contain carbon are organic. A few exceptions are carbon dioxide, calcium carbonate (chalk), and sodium cyanide. These are considered inorganic.
(2) Jones reagent is made up of chromium dioxide, sulfuric acid, and acetone. It is a good idea to avoid getting it on your skin.
(3) The azo group (nitrogen-nitrogen double bond) is frequently associated with color, especially if it is part of a long chain of conjugated atoms. Many dyes contain azo groups.
(4) This is no ordinary ketone. The trifluoromethyl group makes it much more reactive.
(5) It's not that simple. There is more going on here than simply the decrease of one conjugated unit. The oxygen atom plays a large role, but explaining this will cause the few of you who haven't already killed yourselves to do so promptly.



