The Frank R. Lautenberg Chemical Safety for the 21st Century Act amends the Toxic Substances Control Act (TSCA) and was signed into law June 22, 2016. It created a mandatory requirement for EPA to evaluate existing chemicals with clear and enforceable deadlines, to do so in a transparent fashion, and to do so using risk-based chemical assessments rather than rely on simple epidemiological correlations.
EPA selected the first 10 chemicals to undergo risk evaluation under the amended TSCA and to make those understandable for the public, the American Council on Science and Health is producing risk-based evaluations of each, which will then be compiled into a free downloadable book for consumers.
What is Trichloroethylene (TCE)?
Trichloroethylene (TCE) is a colorless, volatile liquid that is nonflammable and has a sweet odor. It evaporates quickly into the air, but can also be found in water and soil. It is used as a solvent to remove greases, oils, fats, waxes, and tars from metal parts, and is also used to clean cotton, wool, and other fabrics. It is also a component of adhesives, lubricants, paints, varnishes, paint strippers, pesticides, and cold metal cleaners. In the past it was used as a food extraction solvent for natural fats and oils, the decaffeination of coffee, and for cosmetic and drug products.
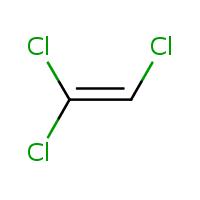
The chemical structure of trichloroethylene is very simple as shown in the graphic; two atoms of carbon in a double bond with 1 hydrogen atom and 3 chlorine atoms. This simple structure and the ability of our bodies to metabolize TCE lead to its principle effect, that of liver and kidney toxicity at high levels of exposure. This is similar to other solvents, including alcohol.
Exposure to TCE
The US Agency for Toxic Substances and Disease Registry (ATSDR) of 2014 states that TCE is commonly found in outdoor air at levels far less than 1 part per million (ppm) but levels as high as about 0.02 ppm have sometimes been measured inside homes and in public places. In workplace air at facilities that use TCE for metal degreasing, it has been measured from 1 ppm up to 100 ppm but it is not chemically stable in air and is broken down quickly. TCE has also been found in drinking water samples in the United States, at levels far less than those found in air, typically less than 30 parts per billion (ppb).
In contrast to air, TCE breaks down slowly in surface water (such as lakes and rivers) and is removed from these waters mostly through evaporation. TCE can also slowly enter groundwater from surface water. In these cases, TCE is expected to remain in groundwater for long periods of time because it is contained and cannot readily evaporate. TCE is also found in soil; it can move through soil to the groundwater or evaporate to air.
Like its fate in groundwater, TCE breaks down only slowly in soil and is removed mostly through evaporation. TCE in soil (and to some extent in groundwater) may evaporate and migrate into air spaces beneath buildings and possibly enter the indoor building air, a process termed vapor intrusion.
TCE Health Effects
TCE is quickly eliminated from the body by breathing, whether exposed occurs through breathing or oral ingestion, find both ATSDR (2014) and EPA (2011). What remains in the body is changed by the liver to other chemicals that are quickly eliminated in the urine. When the body absorbs more TCE than it can eliminate quickly, some of it is stored in body fat. However, once exposure ceases, TCE and its breakdown products are quickly eliminated as above.
Like exposure to any chemical, toxicity of TCE depends on the level to which one is exposed and the length of time of exposure. Both ATSDR (2014) and EPA (2011) report that environmental monitoring data suggest TCE levels, which the public might encounter by direct contact or through air, water, food, or soil, are generally much lower than the levels at which adverse health effects are elicited in experimental animal studies. However, some drinking water sources and working environments have been found to contain levels of TCE that may cause health problems in humans.
Exposure of experimental animals over a few weeks to high levels of TCE result in harmful effects on the nervous system, liver, respiratory system, kidneys, blood, immune system, possibly heart, and body weight loss. Highly controversial evidence exists which concluded that TCE may also cause fetal heart effects (see section on controversy below). Similarly, exposure to modest levels of TCE in people over a few weeks may cause headaches, dizziness, and sleepiness. Larger amounts of TCE may cause nervous system effects related to hearing, seeing, and balance, changes in heartbeat rhythm, liver damage, kidney damage, coma and even death. Some people who get concentrated solutions of TCE on their skin develop rashes.
Both ATSDR (2014) and EPA (2011) note that exposure of several years in experimental animals have also shown liver and kidney damage, immune toxicity, and cancer of various organs. Also noted by these agencies is that TCE exposure of several years in the workplace may cause autoimmune disease in some workers, and some men occupationally-exposed to TCE showed decreases in sex drive, sperm quality, and reproductive hormone levels. In addition, some evidence exists that TCE can cause kidney, liver, or blood cancer in people.
TCE Safe Levels
The federal and state governments develop regulations and recommendations to protect public health. Regulations and recommendations are often expressed as a safe or virtually safe level, that is, a level of a substance in air, water, soil, or food that is not expected to cause any adverse health effect, even in people who are sensitive to the chemical’s effects. These safe levels are usually based on information from experiments with animals (usually rodents) at much higher levels of the chemicals than humans would typically encounter. The higher animal exposures are used to see what the adverse health effects could be. The scientists then conjecture what the adverse effects possibly could be in humans at a lower level of exposure. Scientists can then estimate what a level probably is that will protect humans.
Sometimes these safe levels differ among federal and state organizations because they used different assumptions for human exposure, different animal studies, or employ methods that differ slightly. Other times these recommendations differ because new science develops that suggests different levels are toxic or safe. ATSDR (2014) and NLM (2018b) give examples of these differing recommendations for TCE among government organizations. Recommendations and regulations are also updated periodically as more information becomes available.
Why Is EPA Looking At This Under The Lautenberg Chemical Safety Act?
EPA scientists are currently looking at the likely routes of exposure to TCE in the environment and will be developing exposure scenarios, or pathways, of how the public comes in contact with TCE. These exposure pathways will then be studied by EPA scientists by comparing the amount of TCE exposure in the pathway to its safe or virtually safe level. If human exposure in the pathway is at or below this safe or virtually safe level, then TCE exposure from the pathway is not considered to be a human health concern.
If exposure is above this safe or virtually safe level, then the pathway might be considered as a possible health concern, though small excesses of the safe or virtually safe dose are seldom cause for concern. Safety levels are developed from conservative assumptions, including the use of safety factors that tend to exaggerate risk and exposure pathways that tend to exaggerate exposure. Still, if it becomes a concern regulations might be developed to lessen the exposure of TCE from this pathway. See also EPA (2018) for specific questions related to the assessment of TCE under the new LCSA.
The assessment of TCE toxicity has led to controversy because not all authorities or government agencies consider TCE to be a known human carcinogen. Rodents are not little people, and TCE has only been shown to definitively cause cancer in experimental animals (see for example, NLM, 2018b). In addition, EPA (2011) accepted a study showing developmental toxicity in the hearts of experimental animals at low doses based on a controversial study of TCE exposure in drinking water that is controversial (Johnson et al., 1998, 2003), in part because other studies in experimental animals showed no such effects, including one study specifically designed to replicate these findings (Fisher et al., 2001).
Additional and ongoing attempts at replicating this controversial study have proved to be difficult since the concentration of TCE cannot be readily maintained in the drinking water, leading to additional questions regarding use of that lone study as the basis for EPA’s safe level.
To seek better answers, the Alliance for Risk Assessment (ARA) was petitioned by the Alliance for Site Closures, a small company in Indiana composed of retired state government scientists, to develop a collaboration of interested groups to review EPA’s non-cancer toxicity assessment of TCE and to improve the understanding of current EPA methods to develop risk assessment values. The resulting range of values included EPA’s current safety standards for TCE, which were based in part on the controversial Johnson et al. (2003) study. The Steering Committee of the ARA, composed almost exclusively of government officials, asked that the collaboration focus instead on building a range in EPA’s risk values, based on EPA methods, but using TCE as an example. The resulting range of values included EPA’s current safety standards for TCE, which were based in part on the controversial Johnson et al. (2003) study. The collaboration team included scientists from a number of organizations, including the ATSDR and the nonprofit organization Toxicology Excellence for Risk Assessment (TERA).
The team developed a method that was independently vetted by a team of risk assessment experts, including EPA scientists, and was published in a peer reviewed journal (Dourson et al., 2016).
REFERENCES
Dourson, M.; Gadagbui, B.; Thompson, R.; Pfau, E.;and Lowe, J. 2016. Managing Risks of Noncancer Health Effects at Hazardous Waste Sites: A Case Study Using the Reference Concentration (RfC) of Trichloroethylene (TCE). Regulatory Toxicology and Pharmacology 80:125-133.
Fisher, JW; Channel, SR; Eggers, JS; Johnson, PD; . MacMahon, KL; . Goodyear, CD; Sudberry, GL; Warren, DA; Latendresse, JR; . Graeter, LJ. 2001. Trichloroethylene, Trichloroacetic Acid, and Dichloroacetic Acid: Do They Affect Fetal Rat Heart Development? International Journal of Toxicology. 20(5): 257-267.
Johnson, P. D., Dawson, B.V., and S. J. Goldberg, 1998. Cardiac teratogenicity of trichloroethylene metabolites. J. Am. Coll. Cardiol. 32:540–545.
Johnson, P.; Goldberg, S.; Mays, M.; Dawson, B. 2003. Threshold of trichloroethylene contamination in maternal drinking waters affecting fetal heart development in the rat. Environ Health Perspectives, 111, 289- 292. http://www.ncbi.nlm.nih.gov/pubmed/12611656
U.S. Agency for Toxic Substances and Disease Registry (ATSDR). 2014. Toxicological Profile for Trichloroethylene. October. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp19.pdf.
U.S. Environmental Protection Agency. 2011. Integrated Risk Information System (IRIS) for Trichloroethylene. National Center for Environmental Assessment. EPA/635/R-09/011F. Washington D.C. Available at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0199tr/0199tr.pdf.
U.S. Environmental Protection Agency. 2018. Technical Fact Sheet – Trichloroethylene. Available at: : https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-trichloroethylene-tce#q3.
U.S. National Library of Medicine. 2018a. ChemIDplus:TCE Toxnet database. Found at: https://chem.nlm.nih.gov/chemidplus/name/tce.
U.S. National Library of Medicine. 2018b. International Toxicity Estimates for Risk (ITER) database. Found at: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~kZPiEp:1
Authors:
Michael Dourson, PhD, DABT, FATS, FSRA
Bethany Hansen, MA.
Patricia McGinnis, PhD, DABT
All with Toxicology Excellence for Risk Assessment (TERA) of Cincinnati, Ohio, a 501c3 nonprofit organization created in 1995 with a mission to protect public health.



