Let's hear what some "experts" in biochemistry and toxicology have to say about formaldehyde:
"I won't bury the lead (1): last week the International Agency for Research on Cancer (IARC), the most prestigious international body for cancer assessment in the world, determined that sufficient evidence exists to link formaldehyde with leukemia, a cancer of the blood or bone marrow. The link with leukemia means that the overall impact of formaldehyde on human cancers is much greater than previously thought."
Jennifer Sass, NRDC (2009)
"The small doses [of formaldehyde] are not insignificant because we are all exposed to chemicals in personal care products every day, several times a day. Those exposures are there in addition to exposures to other toxics in food we eat, water we drink, air we breathe and so forth. All of these exposures add up, and could lead to diseases later in life."
Environmental Working Group Website (2009)
"Paint, furniture and cleaning products [in homes] degrade the quality of the air inside because they are often made with toxic materials such as formaldehyde."
Deepak Chopra (2017)
Could you possibly find a worse source of science than NRDC, EWG, or Deepak Chopra?
"Good luck with that"
Josh Bloom, ACSH
So, it will be especially hard for the above "experts" to grasp what I'm about to tell you - not only is your body capable of handling much larger quantities of formaldehyde than you'll ever get from furniture, a soda, or shampoo, but formaldehyde is actually required for cell growth in all living cells. You would be somewhat dead without formaldehyde because of a biochemical pathway called the one-carbon cycle, which uses endogenously produced formaldehyde - and a whole lot of it - to make the essential biomolecules amino acids and DNA.
The one-carbon cycle, which was described in a 2017 Nature paper (2), is a pathway that is responsible for creating, using, and disposing of formaldehyde. Senior author Dr. Ketan Patel, of the MRC Laboratory of Molecular Biology, explains (emphasis mine):
"We've discovered that some [the formaldehyde] comes from an unexpected source, a key pathway - called the one carbon cycle - that's used to make the building blocks of life, such as DNA and certain amino acids. The one-carbon cycle is a fundamental process which is present in all forms of life, right down to bacteria."
The pathway is rather complicated. Here it is in simplified form (Figure 1). (3)
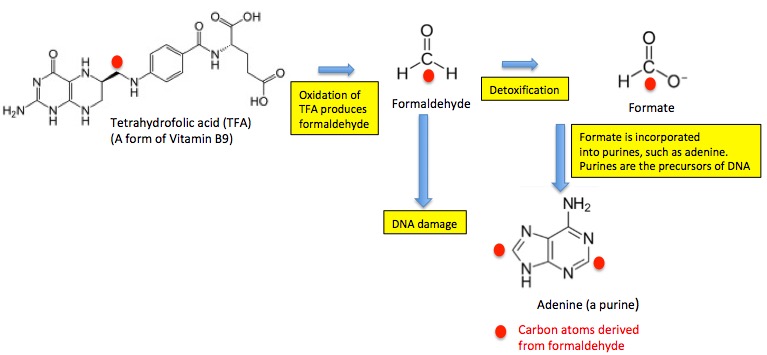
Figure 1. A simplified view of the one-carbon cycle. Formaldehyde is produced in the body by enzymatic oxidation of Vitamin B9. Once produced it can be detoxified by either of two biochemical reactions, one of which converts it to formate, which is then used to make the DNA and amino acids that cells require. No formate, no DNA.
Two important sources of endogenous (produced internally) formaldehyde are the amino acid serine (4), and tetrahydrofolic acid (TFA), a form of Vitamin B9. Let's focus on the latter.
TFA is oxidized by a liver enzyme. In the process, the carbon atom which is marked by a red oval (Figure 1, left) is converted into formaldehyde. There are two biochemical mechanisms used to detoxify formaldehyde; one is conversion to non-toxic formate by an enzyme called formaldehyde dehydrogenase. The folate that is formed is then incorporated into biomolecules called purines, which are the building blocks of DNA.
The proof of this incorporation was demonstrated by feeding 13C labeled formaldehyde to cultured cells and then seeing in which molecule it ended up. One place was adenine, one of the four DNA bases (Figure 2).
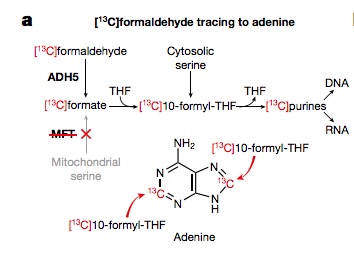
Figure 2. When 13C-labeled formaldehyde is fed to cells it can be traced by analyzing adenine to see whether it is incorporated into the molecule and, if so, where. Isotopic labeling a standard method of determining mechanisms. Source: Patel, et. al. Nature, 548(7669), 549–554 (2017)
So, how much formaldehyde are we talking about? A whole lot. A 2014 report from the European Food Safety Agency EFSA) entitled "Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources" estimates that the human body makes and processes about 50 grams (50,000 mg (!)) of formaldehyde every day.
Endogenous turnover of formaldehyde was estimated to be approximately 0.61‐0.91 mg/kg bw per minute and 878–1310 mg/kg bw per day (5) assuming a half-life of 1.5 min.
Let's compare that 50 grams to the amount of formaldehyde you might ingest from other sources:
- One packet of aspartame is converted to 10 mg of formaldehyde (0.02% of 50 grams) (See Sorry, Your Diet Coke Is Not Calorie-Free)
- A pear (16 mg, 0.03%)
- One kilogram of coffee (4 mg, 0.0075%)
Do you see how ridiculous these formaldehyde scares are? The scientists at the EFSA sure do (emphasis mine):
"At the current [acceptable daily intake] of 40 mg/kg [body weight] per day for aspartame, formaldehyde would be approximately 4 mg/kg [body weight] per day and represent only 0.3‐0.4 % of the endogenous turnover of formaldehyde."
The fact that a chemical that is genotoxic (and also just plain toxic) but is also required in all living cells, speaks volumes about the way our bodies are engineered to make, use, and protect ourselves from formaldehyde. The ability of our livers to detoxify a wide variety of chemicals explains we aren't all dropping dead from the multitude of chemicals to which we are routinely exposed.
Time to find another phony chemical scare, EWG. This one is stale. Probably wasn't preserved properly. I hear formaldehyde is pretty good for that.
NOTES:
(1) Perhaps Sass should bury her Ph.D. in biology instead.
(2) Burgos-Barragan, G., Wit, N., Meiser, J., Dingler, F. A., Pietzke, M., Mulderrig, L., … Patel, K. J. (2017). Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature, 548(7669), 549–554. doi:10.1038/nature23481
(3) Here it is in non-simplified form. Enjoy yourself.
The "I wanna kill myself" version of the one-carbon cycle. Source: Wikipedia
(4) Serine is also converted to formaldehyde in a biochemical reaction involving Vitamin B6 (pyridoxal phosphate) and an enzyme called serine hydroxymethyltransferase.
(5) Based on an average body weight of 60-70 kg (132-154 pounds) 878–1310 mg/kg body weight per day comes out to about 50-75 grams of formaldehyde.




