Chemists have a variety of reasons for synthesizing things. Sometimes it can be purely academic or exploratory. Other times it can be to provide life-saving drugs (See: Semisynthetic: A *Real* Word That Saves Lives"). Often, research groups will compete to be the first to synthesize a particular molecule. And others will try to provide practical solutions, such as discovering new materials for semiconductors. One rather obscure niche involves finding better explosives.
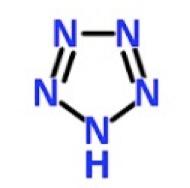
For more than a century, maniacs have been trying to synthesize a simple chemical called pentazole—a substance that is so unstable that it blows up even when you draw the structure on paper.
But, a Chinese group just became the first to make it. In a paper just published in the journal Science, Zhang Chong and colleagues described how to make a substance that has eluded others for decades.
They used a very clever trick to pull it off. First, a little about explosives. Virtually all explosive molecules have one or more things in common:
- They have a very high proportion of nitrogen or oxygen to carbon in the molecule.
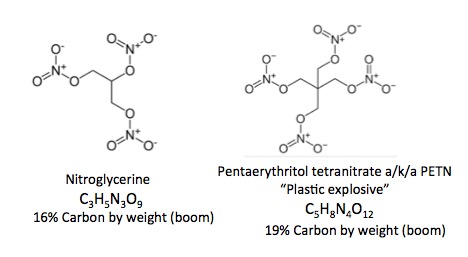
- They have gases, usually nitrogen or oxygen, that are trapped within an unstable organic molecule. The six oxygen atoms in TATP are dying to get out of there. So much so, that if the material is dry, tapping it with a spoon will detonate it.
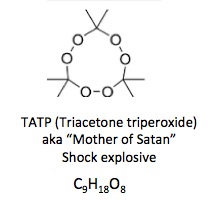
3. They contain no carbon at all. Just gasses, usually nitrogen and oxygen. These are only too happy to escape when given a nudge.
(See: "Why Stuff Explodes," and "Blowing Up Your Lab Mate Is A Bad Idea, But It Sure Is Fun.")
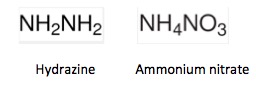
Hydrazine is used as rocket fuel. Ammonium nitrate destroyed an entire city in Texas in 1947.

So, it should be of no surprise that N5 was so unstable and tough to make. Those five nitrogens that make up the pentazole ring want nothing to do with each other. So much so that they refused to exist in the same molecule until now.
Cranky nitrogens. Image: ChemSpider
The Zhang group needed a little help keeping the thing together, which sort of explains the complexity of the title:
"Synthesis and characterization of the pentazolate anion cyclo-N5ˉ in (N5)6(H3O)3(NH4)4Cl"
Think of pentazole as an Oreo cookie. Everyone knows that the stuff in the middle cannot exist on its own. It needs the cookie part to hold it together.
The scientists who managed to make pentazole also needed a little help holding it together. Instead of a cookie, they used water and ammonium chloride in precise amounts, and crystallization techniques to form a stable, crystalline complex having the formula (N5)6(H3O)3(NH4)4Cl. Perhaps even more impressive is the structure of the complex, which was solved by X-ray crystallography.
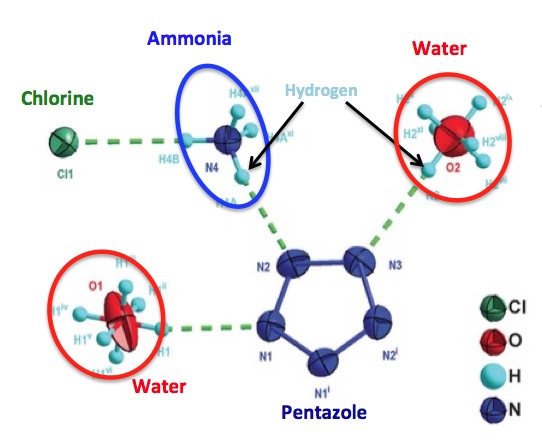
Molecular interactions required to "tame" pentazole. The green dashes are called hydrogen bonds. Three of the five nitrogen atoms (dark blue) in pentazole are stabilized by hydrogen bonds—two from the hydrogen atoms in water (red circle) and one from ammonia (blue).
Adapted from Science 355, 374–376 (2017) 27 January 2017
The complex itself, which contains five pentazole molecules, looks like this: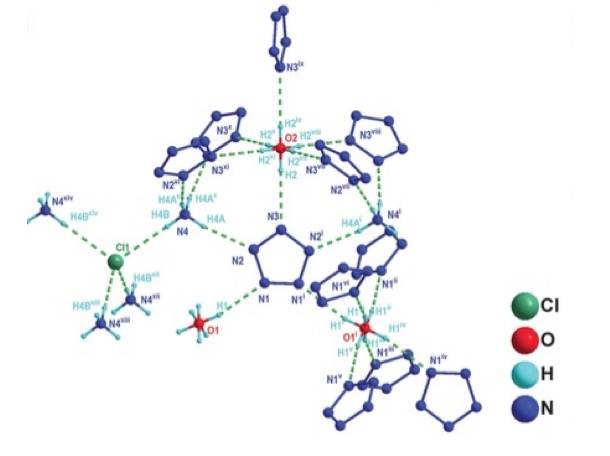
The chemical structure of (N5)6(H3O)3(NH4)4Cl—the first isolated form of pentazole (1).
Perhaps the best part of the paper is: "(N5)6(H3O)3(NH4)4Cl was found to be surprisingly stable, with a decomposition onset temperature of 117°C." I believe this is the origin of the expression "here, you do this." And another one that I shall not repeat, bit it begins with "F."
Bang up job, guys
Note:
(1) The structure of the complex contains five pentazole rings, but the figure about shows ten. This is an artifact. Each ring is shown in two positions, each representing the probability the ring being one of two locations.
Original citation: Zhang et al., Science 355, 374–376 (2017) 27 January 2017



