
If someone is going to commit mass murder of innocent civilians, it's not too much of a stretch to assume that he's going to lie about it. Which is exactly what Bashar al-Assad did when he denied that Syria used chemical weapons in the April 4th attack in the town of Khan Sheikhoun in northern Syria.
There was never much question that the chemical agent used was Sarin gas (1), but since Sarin is Sarin, isn't it at least plausible that someone else used the neurotoxin? Is it fair to pin it on Assad?
Actually, it is. This is because Sarin is Sarin, but without a second chemical called a stabilizer, the molecule is too unstable to keep around; the byproduct of Sarin is hydrofluoric acid, which catalyzes its decomposition. In order to neutralize the acid and preserve the toxin, a class of basic compounds called amines is used. Different groups and countries use different amines as stabilizers, which provides a "Sarin fingerprint." In this case the finger points directly at Assad since Syria has been known to use a stabilizer called hexamethylenetetramine (aka hexamine), which was detected long after any trace of Sarin remained (2).
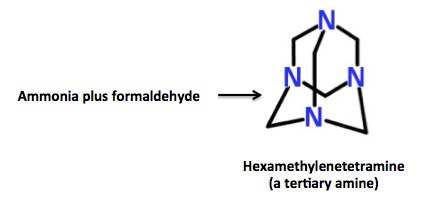
Although an amine is needed to stabilize Sarin, not any amine will do the job. This is because most amines will react quickly with Sarin, and convert it to something that is non-toxic. Please forgive the following chemistry lesson:
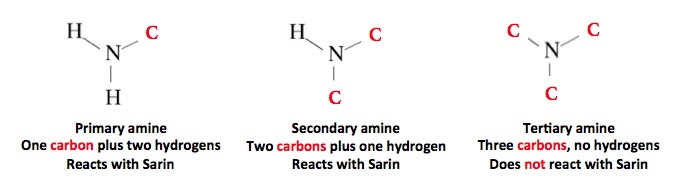
The three classes of amines. Tertiary amines are unreactive, so they can be used to stabilize Sarin without destroying it.
Bashar al-Assad's Sarin "recipe" actually made it easier for scientists to pin this act on him. There are only a handful of tertiary amines that have been used to stabilize Sarin, and hexamethylenetetramine—a stabilizer that can be traced directly to Syrian production facilities— is one of the easier amines to track. Here's why.
Triethylamine, a very common lab chemical, is one of the other tertiary amines that has been used to stabilize Sarin. It works perfectly well as an acid scavenger, but unlike hexamethylenetetramine, you'll never find it later on. This is because of one of its physical properties—it evaporates very quickly.
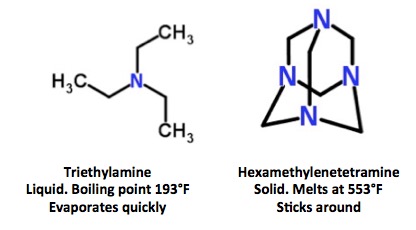
Triethylamine and hexamethylenetetramine are both tertiary amines, which can be used to stabilize Sarin. Triethylamine evaporates almost immediately. Hexamethylenetetramine does not.
So, by choosing hexamethylenetetramine to stabilize (3,4) the Sarin, the Syrian scientists who made it left a "chemical calling card" that gave away its origin. Perhaps Bashar al-Assad just doesn't care, since everyone on Earth knew that he did it anyhow. Or maybe he's just a bad chemist. And a far worse person.
Notes:
(1) The symptoms that affected the victims were consistent with the use of an organophosphate nerve agent. Sarin and VX are the poisons "of choice." It had to be one of them.
(2) Sarin is not only volatile but highly chemically reactive. It decomposes rapidly under various sets of conditions. Within a week (and probably far less) after the attack, none would remain.
(3) Hexamethylenetetraamine is a very good acid scavenger. It is a white solid that is easy to handle, and it has four basic nitrogen atoms in the molecule. Each nitrogen will neutralize one molecule of hydrofluoric acid, so you need to use four-times less of it.
(4) Since hexamethylenetetraamine is used for other applications, for example, manufacturing plastics, its detection is not a guarantee that is was used by Bashar al-Assad in the attack, but it's damn close.



