
There are unquestionably elements that are rarer, stranger, and more exciting than phosphorous. But the element, which sits under nitrogen in Group 5A on the periodic table, forms chemicals that are responsible for all life, and others that provide a very nasty death. And it forms pretty minerals. If this sounds like foreplay for a chemistry lesson, you got that right, Daddio.
First, Pretty Pictures

Phosphate minerals are usually composed of phosphate and a variety of metals. Photo credits (left to right) Wikipedia, Wikipedia, Matt Zukowski/Mindat.org
Now...
The Dreaded Chemistry Lesson From Hell® (Don't complain. You KNOW you want this.)

Photo credit: Wikimedia Commons
Elemental Phosphorus (0) - Three Personalities
Elemental phosphorous is too reactive to be found in nature; it is primarily found as minerals of phosphate PO43– its most stable, oxidized form. But phosphate can be converted to elemental phosphorous by simple chemical reactions. Elemental phosphorous is rather unique in that it exists in different crystaline forms, called allotropes, each with different properties and different colors.
White (photo below, left) ignites in air, Red (center) is used for matches, Black (right) is used for pretty much nothing, with the possibility of the following:
"To prepare black phosphorus quantum-dots (BPQDs) and nano-platelets by liquid-exfoliation"
[Disclaimer: I'm not as smart as you might assume. I read this quote a few times. In total, I understand the terms "to" and "and." Somehow, these guys, who presumably have an odd number of chromosomes, do.]
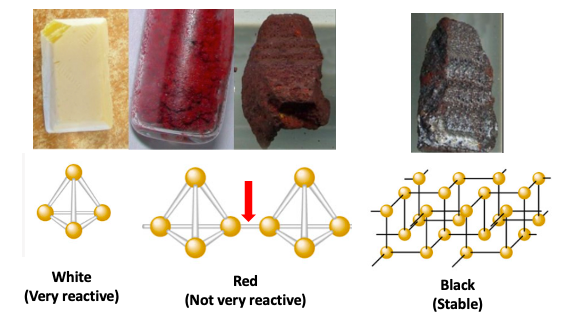
Photo: Wikipedia. Beneath the photos are the chemical structures of the allotropes. Note that as we go from left to right the phosphorous atoms become more interconnected and the stability increases. (This is a common property of chemicals.) The red arrow shows the additional bond that forms when white phosphorous is heated and becomes the red form.
Phosphorous (V) - Life and Death
Life
Perhaps the most important function of phosphorous is (as phosphate) holding the pieces of DNA and RNA together to make genes. No other element possesses the properties to form chains of phosphate esters – the so-called "backbone" of DNA/RNA. It would take an entire chapter to fully explains this; I will cheerfully hang myself before writing it. So, you're just going to have to believe me or ask The People's Chemist, who I recently trashed, to explain. You may need to wait until he looks up the definition of "phosphorous," but that's just snarky speculation.
One interesting factoid: Phosphorous is the 13th most abundant element in the earth's crust (0.099%), sandwiched between fluorine and manganese, but Mother Nature chose it as the basis of all genetic information (and thus, protein synthesis) because of certain properties, for example, the strength of a phosphorous-oxygen bond that helps keep DNA/RNA intact, or the ionizable O-H bond (because of its ability to exist in the +5 oxidation state) that makes DNA/RNA more easily soluble in water.
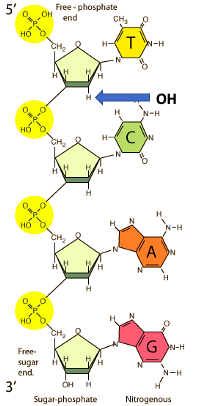
Figure 1: Four nucleotide units (nitrogenous base-sugar-phosphate) connected together by phosphate ester bonds (yellow circles). The blue arrow is pointing to the position on the sugar that determines whether the genetic material is RNA (OH is there) or DNA (H is there). Source: QUANTUM MECHANICS AND CANCER BIOLOGY
Death
Phosphorous giveth and taketh away. There are a bunch of examples of how toxic phosphorous is, but I'm going to focus on the organophosphate nerve gases (1) because these bad boys are some of the most poisonous chemicals on earth. Ironically, it is their ability to form phosphate esters that makes these bad boys so deadly.
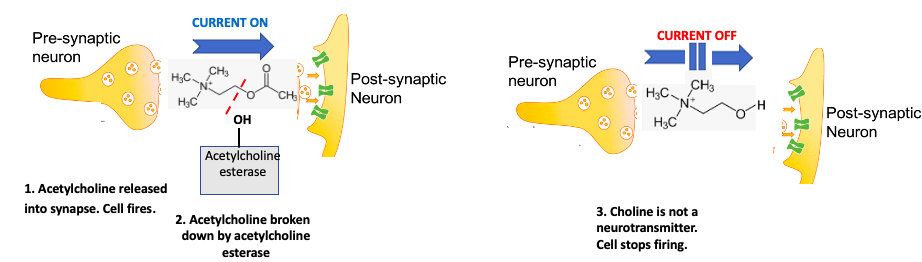
Figure 1. Normal nerve cell function. (Left) The "left side" of the neuron (pre-synaptic synapse) releases the neurotransmitter acetylcholine, which binds to the post-synaptic neuron, causing the cell to fire. Then, the enzyme acetylcholinesterase breaks down the acetylcholine (red hatch line) to choline. (Right) Choline is not a neurotransmitter; the current is interrupted and the cell stops firing. The acetylcholine-choline interconversion can be thought of as a chemical switch. Original image: IEEE
Sarin, and other organophosphorus neurotoxins, screw up the chemical switch (Figure 2). The hydroxyl (OH) group (which is part of the amino acid serine) is critical for breaking down the acetylcholine in the synapse. When the hydroxyl group is unavailable the enzyme is incapacitated (the switch stays on). Since acetylcholine controls muscle contraction, muscles will contract and stay that way in the absence of a viable enzyme to break it down. This is why Sarin, VX, and similarly lovely concoctions are so deadly. Sarin (shown below) reacts with the hydroxyl group of acetylcholinesterase forming a stable phosphorous-enzyme complex, taking the OH out of commission (2).
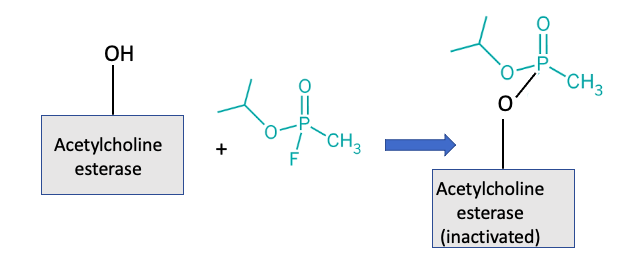
Figure 2. Nerve cell function interrupted by Sarin. Sarin reacts with the hydroxyl group of acetylcholinesterase, tying it up as a stable phosphorous-enzyme complex. In the absence of functional acetylcholinesterase, the on-off switch stays on and the nerve keeps firing. This is responsible for the deadly neurotoxic effects of the chemical.
I've run out of ATP molecules (also the basis of life) aka energy, so it's time for lunch. Hope all both of you readers enjoyed the lesson.
NOTE:
(1) I could have also gone with phosphine (PH3), a hideous gas that killed a bunch of people in Texas, something I wrote about in 2017. I don't know a single chemist who has ever used it.
(2) A more comprehensive discussion of Sarin and VX neurotoxins can be read here.



