Microbiologist Stanley Falkow is credited with saying, "The goal of every bacterium is to become bacteria." Similarly, the goal of every pathogen is to infect a new host.
Pathogenic microbes face an evolutionary trade-off: On the one hand, they want to be as nasty as possible, because the nastier they are, often the easier it is to spread from one host to the next. Think of Vibrio cholerae, the causative agent of cholera, which upon infection can result in a person producing gallons of diarrhea. Death is due to dehydration, but not before the patient served as an incubator and excreted billions of more bacteria into the environment.
On the other hand, a microbe does not want to be too nasty. If it incapacitates or kills its host quickly, it will be difficult for the microbe to spread to another host. So another strategy is a long-term infection, an extreme version of which is employed by herpes viruses. After a passionate one-night stand, the love might be gone the next day, but herpes will live with you forever. The virus mostly lays dormant inside of infected cells, only occasionally causing "outbreaks." The host is never too sick but can reliably spread the virus1.
In other words, a pathogenic microbe is like a morbid version of Goldilocks: It wants to get its virulence "just right."
Ebola: Evolving to Become More Deadly?
 From 2013 to 2016, western Africa faced an enormous outbreak of Ebola in which 28,616 people fell ill and 11,310 died. The overall case-fatality rate (i.e., the percentage of people who died from the infection) was nearly 40%. However, the case-fatality rate varied greatly. In some locations, the virus killed 80 to 90% of the people it infected. Why?
From 2013 to 2016, western Africa faced an enormous outbreak of Ebola in which 28,616 people fell ill and 11,310 died. The overall case-fatality rate (i.e., the percentage of people who died from the infection) was nearly 40%. However, the case-fatality rate varied greatly. In some locations, the virus killed 80 to 90% of the people it infected. Why?
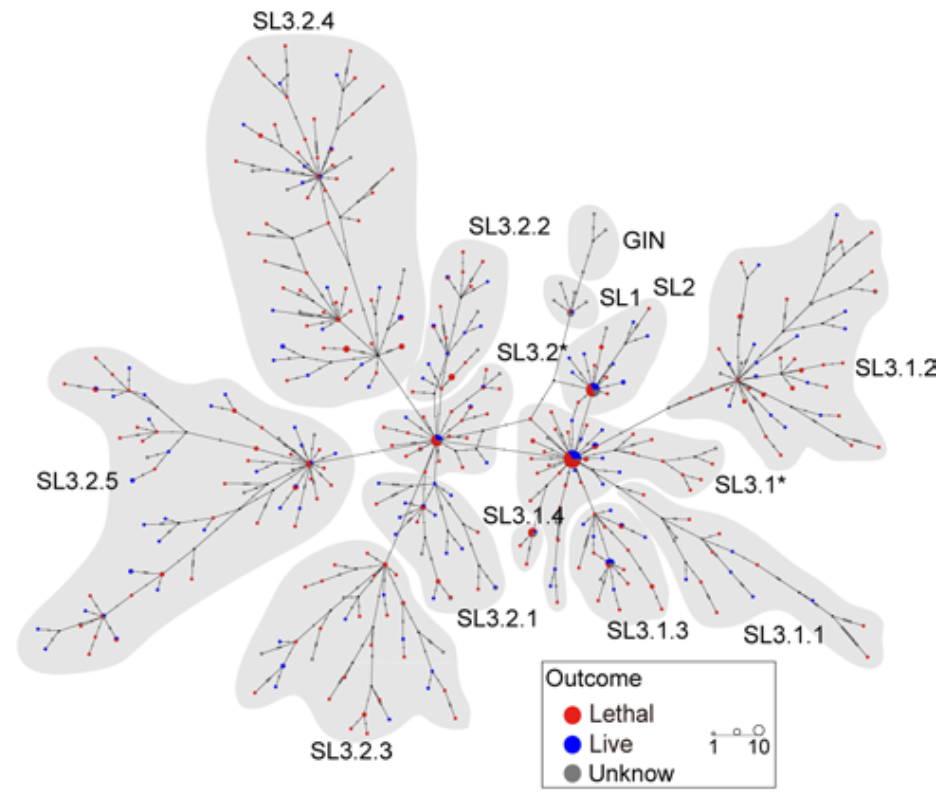
The virus was evolving into many different lineages, a new study suggests. (See image on right.) An international team of mainly Chinese researchers sequenced and analyzed the genomes of Ebola viruses sampled from 514 people in Sierra Leone in 20142. As the virus spread, it was gathering mutations. By tracking these mutations, the researchers constructed a "family tree," which consisted of 11 distinct lineages.
When the team linked their Ebola genetics data with patient outcomes, they found that some mutations made an Ebola lineage more lethal, while other mutations made a lineage less lethal. (See diagram below.)
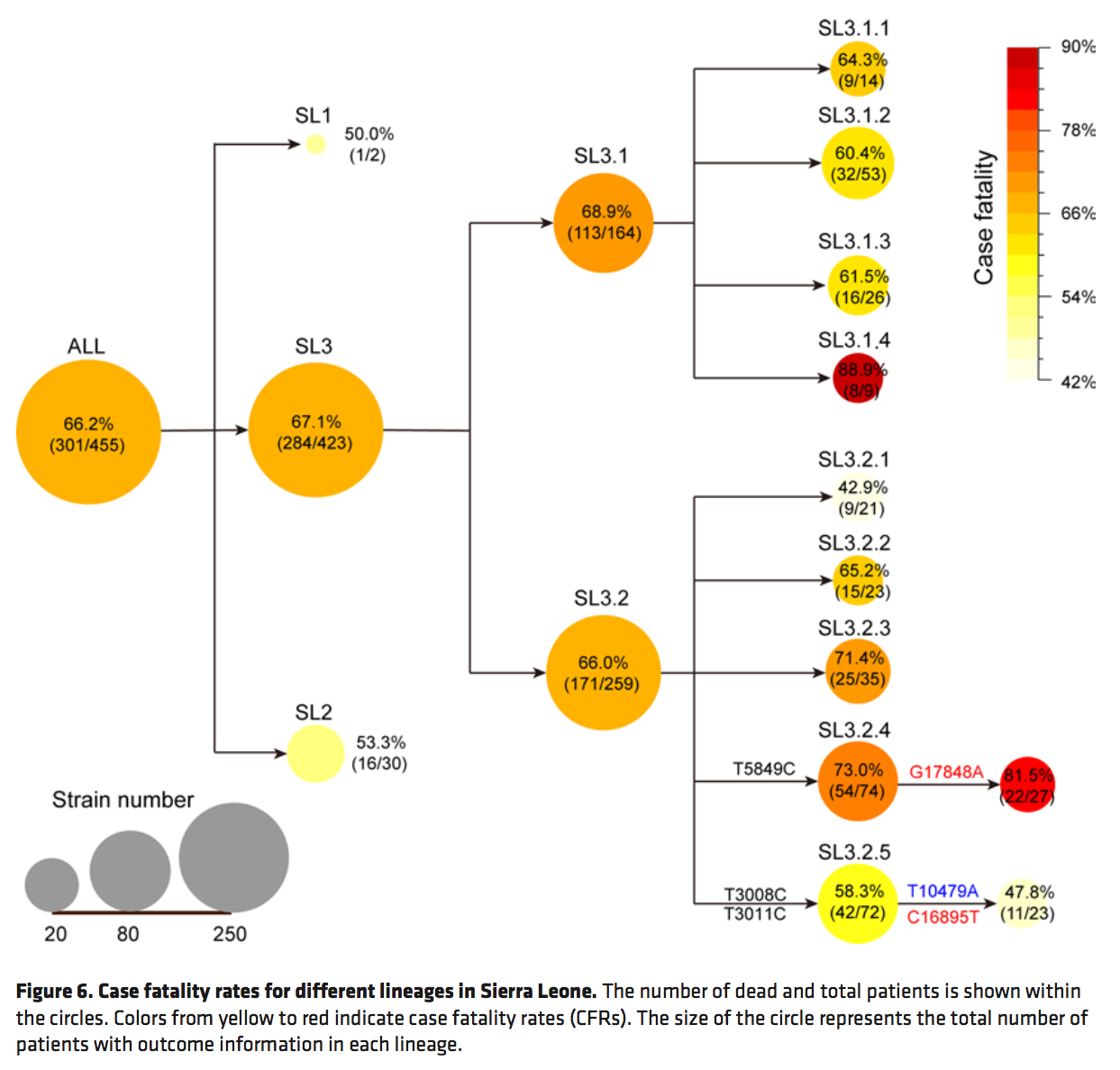
Take lineage SL3.2, for instance. The case-fatality rate was 66%. But when this virus picked up a mutation called T5849C, the case-fatality rate increased to 73%. When the virus gained yet another mutation, called G17848A, the case-fatality rate leapt to 81.5%. Clearly, viral genetic factors played a key role in determining whether a patient lived or died.
A Key Limitation and a Creepy Conclusion
Studies like this are limited by the researchers' inherent inability to do "random sampling." In an outbreak, scientists tend to collect whatever data they can, which means that their sample may not be representative of all Ebola strains. Indeed, that was almost certainly true in this study, since the authors had an overall case-fatality rate of 66.2%, which was considerably higher than the international outbreak as a whole (which was about 40%).
Still, much can be concluded from their data, such as an indication as to which mutations may make Ebola more or less deadly. Additionally, the authors' genetic analysis suggested that people who died from Ebola possibly spread the virus to more people than those who survived. They are uncertain why this would be the case, but they suggested that perhaps those who died had a greater number of viral particles in their bodies or were touched by a greater number of people (e.g., for burial).
Regardless of the exact explanation, the possibility that natural selection might favor Ebola evolving to become more lethal is troublesome.
Notes
(1) Herpes is most infectious during outbreaks, but a person can still transmit the virus when he or she is symptom-free.
(2) The clinical outcome (i.e., whether the patient lived or died) was known for only 455 patients.
Source: Tao Li, et al. "Mapping the clinical outcomes and genetic evolution of Ebola virus in Sierra Leone." JCI Insight. 2 (15): e88333. Published: 3-Aug-2017. doi:10.1172/jci.insight.88333.