The chemistry of gasoline is not as simple as you'd think. In the absence of additives, your engine will knock itself into oblivion (See: Octane Rating And Lead: Explaining The Chemistry Of Gasoline). Over the years, a number of anti-knocking additives have been used, including one that, in hindsight, was a terrible idea: tetraethyllead (the lead in leaded gasoline). More recently methyl t-butyl (MTBE) was used until it was discovered that the chemical was water soluble (duh) and accumulated in groundwater. Tetraethyllead was banned in 1996 and MTBE hasn't been used since 2005.
At this time almost all cars use ethanol (10%) as the additive to prevent engine knock (1), however, not without environmental controversy or technical problems. For example, ethanol is corrosive to engines, and also hygroscopic (absorbs water). So when a 10% ethanol-gasoline blend (aka E-10) sits around for a while it will also absorb water from the air. Once the amount of water in the blend reaches 0.5% (this takes about 90 days), the ethanol will contain enough water so that it becomes insoluble in gasoline and will separate into two layers (this is called phase separation). It takes only one tablespoon of water in a tank of gas to cause this to happen. Not good for your engine. It looks like this:

Phase separation of wet ethanol and gasoline. Photo: icbamarketing.com
Since gasoline without an antiknock agent will ruin your car, something has to be used. A group of chemists at the University of Bristol has come up with an interesting solution: beer. Not beer itself, but rather a chemical called isobutanol, which is a component of the fermentation mixture of beer. Isobutanol is more lipophilic (fat loving) than ethanol, which makes the solubility problem dissolve (sorry about that one), nor is it hygroscopic, both of which make it a better gasoline additive. But unlike ethanol, which can be produced by fermentation of corn or cellulose, there is no good source for the stuff.
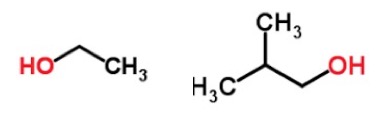
The chemical structures of ethanol (left) and isobutanol. Source: ChemSpider
The Bristol group discovered a method for converting ethanol into isobutanol. This isn't as bad as it sounds:
"Our group has investigated converting readily available (bio)ethanol to n-butanol using homogeneous ruthenium catalysts with either P-P bidentate or mixed donor P-N ligands, achieving excellent selectivity at good conversion for this transformation."
Pellow, et.al., Catal. Sci. Technol. (2017)
OK, that's a lie. It really is as bad as it sounds, and it looks even worse.

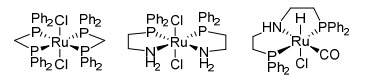
Three catalysts used to convert ethanol to isobutanol. Pellow, et.al., Catal. Sci. Technol. (2017)
Those three hideous looking structures above are catalysts - chemical reagents that promote the conversion of one chemical compound to another. One common type of catalyst is called an organometallic complex. These contain a central metal atom bound to certain atoms (these are called ligands). In this case, the central atom is ruthenium, and it is bound to phosphorous, nitrogen, chlorine or carbon monoxide (this is called a metal carbonyl complex). PPh2 refers to a phosphorous atom with two benzene rings attached. You are unlikely to find this at Home Depot.
The conversion of ethanol to isobutanol has been accomplished using pure anhydrous (containing no water) ethanol. But the challenge is getting this reaction to work in the presence of water, which often causes organometallic catalysts to break down. So, the Bristol group is using beer as a testing media to find catalysts that will perform this reaction under conditions that simulate those in fermentation broths.
"The alcohol in alcoholic drinks is actually ethanol - exactly the same molecule that we want to convert into butanol as a petrol replacement. So alcoholic drinks are an ideal model for industrial ethanol fermentation broths - ethanol for fuel is essentially made using a brewing process."
Duncan Wass, Ph.D., School of Chemistry at Bristol University
The synthesis of isobutanol is an important problem, both for environmental reasons and energy production (2). Let's raise a toast and wish them luck.
NOTES:
(1) When "low-octane" gas is blended with 10% ethanol aka E-10 its octane rating is boosted to 87 (standard gasoline).
(2) Isobutanol is not only better for car engines. It also contains more energy than ethanol, making it a better additive.




