These days all that is green is good. Perhaps even holy. Cars, milk cartons, grocery bags, dry cleaners, cat litter. You name it, it's green. But here's an exception - potatoes. You might want to pass on the green potatoes. A report (1) by the Federal Institute for risk assessment (BfR), which is the German equivalent of a combination of our FDA, EPA, and USDA of a poisoning of a German family was just issued and documented a 2015 case of poisoning from a potato dish.
While one case in 3+ years hardly constitutes an epidemiological emergency, it is the chemistry that is responsible for the toxicity and the color which is what is really interesting. And there's a lesson too (vegans - pay attention): Plants do not exist to be eaten - quite the opposite. They exist to survive and propagate. This is why so many plants produce toxic chemicals - as a defense mechanism. In the case of potatoes, the culprit is a glycoalkaloid (2) called solanine (Figure 1), a chemical you don't want to mess with. A dose of 200 mg - 0.007 ounces - can be fatal.
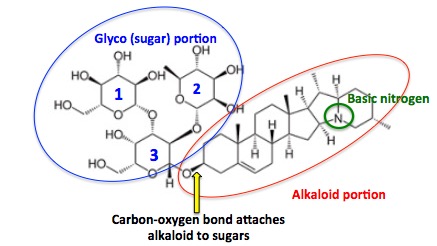
Figure 1. The chemical structure of solanine. (Left) Three sugar molecules (blue circle, blue numbers) are chemically bound to each other, forming a trisaccharide - a chemical consisting of three sugar molecules. The sugars are responsible for the "glyco" part of the glycoalkaloid. The trisaccharide is attached to the alkaloid (aglycone (3)) half of the molecule (Right, red circle) by a carbon-oxygen bond (yellow arrow). The basic nitrogen, which is common to all alkaloids is indicated by the green circle.
Now it's time to admit that the title was a lie. Sort of. Solanine is not green, yet green potatoes can dangerous. What is going on? It's Mother Nature playing a little trick on us. The green is actually chlorophyll, which is certainly not harmful (unless it's in kale, then all bets are off.) So, something else must be going on. It is.
What we are seeing is the result of two temporally and mechanistically linked, yet independent events. You don't need a degree in botany to know how chlorophyll is formed, but it just so happens that the conditions that make chlorophyll are the same as those responsible for the biosynthesis of solanine - exposure to light. Solanine biosynthesis in potatoes is an adaptive protective mechanism; the potato makes it in response to attack by insects (other organisms) or if the skin is broken. Or if the potato gets a healthy dose of light. Chemicals like solanine, which produce protective toxins in response to light (among other challenges to the plant) are called phytoalexins. There are thousands of them found in nature.
So, what is really going on are two processes going on at the same time, and caused by the same stimulus, but independent of each other. In this case, the green from the chlorophyll is acting as a surrogate marker for the presence of solanine. How cool is that??
One final factoid. If potatoes have this little trick up their potato-sleeves, then it is a pretty sure bet than other plants have figured this out. They sure have. Potatoes are a member of the nightshade family of plants. You know, like deadly nightshade. Another name for this family is Solanaceae, so the name solanine is not arbitrary.

Deadly nightshade. Beautiful and dangerous (4). Photo: Minnesota Wildflowers
Here are a few other members of the nightshade family:
- Bell peppers
- Tomatoes
- Eggplant
- Tobacco
- Okra
- Gooseberry
Important information for the nascent anti-vegite movement. I am proud to be a member. Those things are icky.
NOTES:
(1) Don't bother. It's in German.
(2) An alkaloid is a basic, nitrogen-containing chemical found in plants. About 4,000 alkaloids have been identified. Most are toxic. Some examples are nicotine, quinine, morphine, the cancer drug vincristine, and lysergic acid (but not LSD. Does anyone know why?) Glycoalkaloids are alkaloids with one or more sugars attached, either via a nitrogen or oxygen bond. They are neurotoxins.
(3) The term "aglycone" is commonly used in natural products chemistry. An aglycone is the portion of the molecule that does not contain the sugar(s).
(4) Glycoalkaloids are neurotoxic because they are inhibitors of the enzyme acetylcholinesterase, the same enzyme that is inhibited by nerve gases, such as VX. There are a few drugs which act by this same mechanism, most notably, the drugs that (supposedly) are helpful for Alzheimer's Disease. One such drug was called Tacrine (Cognex). Aside from the fact that it didn't work and made people sick as hell, it was a fine drug. Tacrine was (mercifully) pulled off the market in 2013 in the US. There are now other crappy AD drugs to take its place.




