The Frank R. Lautenberg Chemical Safety for the 21st Century Act amends the Toxic Substances Control Act (TSCA) and was signed into law June 22, 2016. It created a mandatory requirement for EPA to evaluate existing chemicals with clear and enforceable deadlines, to do so in a transparent fashion, and to do so using risk-based chemical assessments rather than rely on simple epidemiological correlations.
EPA selected the first 10 chemicals to undergo risk evaluation under the amended TSCA and to make those understandable for the public, the American Council on Science and Health is producing risk-based evaluations of each, which will then be compiled into a free downloadable book for consumers.
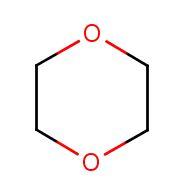
What is 1,4-Dioxane?
1,4-dioxane is a clear solvent with a mild pleasant fragrance used in the manufacture of other chemicals, a laboratory reagent, a trace contaminant found in cosmetics, detergents, and shampoos, and is found in some foods, either naturally occurring or as a contaminant. The structure is very simple; two molecules of ethanol joined together in a ring as seen in the graphic.
This simple structure and the ability of our bodies to metabolize 1,4-dioxane can lead to liver toxicity, it’s principal effect, with high enough exposure, much like other solvents, including alcohol.
1,4-dioxane can be released into the air, water, and soil at places where it is produced or used as a solvent. However, 1,4-dioxane does not remain in the soil, but rather moves into groundwater easily, where it is stable and does not break down quickly. According to both ATSDR (2012) and EPA (2017), the most likely source of exposure to humans comes from water or foods, whether contaminated or naturally-occurring. Current levels of 1,4-dioxane in water are not known, but in the 1970s, the level of 1,4-dioxane in drinking water was 1 microgram per liter of water (1 μg/L). Traces of 1,4-dioxane may also be present in some food supplements, foods containing residues from packaging adhesives, or in food crops treated with pesticides that contain 1,4-dioxane. Many products on the market today (such as pharmaceuticals, cosmetic products, and detergents) may also contain 1,4-dioxane in trace amounts, so human exposure to 1,4-dioxane may occur from these products.
There are no reliable data on amounts, just broad ranges. ATSDR reported that from 1992–1997 the average concentration of 1,4-dioxane in a few cosmetic products reportedly ranged from 14 to 79 ppm while the activist group Campaign for Safe Cosmetics survey results more recently said the levels of 1,4-dioxane in cosmetic products were 1.5–12 ppm in baby and children’s products and 2–23 ppm in adult products.
1,4-Dioxane Health Effects
ATSDR (2012) and EPA (2017) report that 1,4-dioxane rapidly enters organisms from any means of exposure (air, water, food, skin) but is quickly metabolized (or broken down) into other chemicals inside most organisms. The chemicals are rapidly excreted, primarily through urine.
Like exposure to any chemical, toxicity of 1,4-dioxane depends on the level to which one is exposed and the length of time of exposure. Environmental monitoring data available suggest that the total levels of 1,4-dioxane to which the general public might be exposed are significantly lower than can cause adverse or harmful health effects in studies with experimental animals.
But there have been reports of eye and nose irritation at low levels of 1,4-dioxane after short-term exposure. There is no link to human cancer. In higher short-term exposures in experimental animals, breathing vapors of 1,4-dioxane both irritates and then causes more severe effects in the nasal cavity and the liver and kidneys. Short-term experimental animal exposure to 1,4-dioxane via drinking water or by skin contact with liquid 1,4-dioxane also affects the liver and kidneys. After longer term breathing or drinking 1,4-dioxane, cancer inside the nose and liver was found in experimental animals.
Some scientists think that 1,4-dioxane may cause cancer in these animals when the level of exposure (or dose) becomes higher than a certain amount or threshold (EPA, 2013; Dourson et al., 2014; Dourson et al, 2017). Many scientists don't consider the cancer findings in rats and mice applicable to the low exposure situations encountered by people (see section on controversy below).
1,4-Dioxane Safe Levels
The federal and state governments develop regulations and recommendations to protect public health. Regulations and recommendations for chemicals are often expressed as a safe or virtually safe level, that is, a level of a substance in air, water, soil, or food that is not expected to cause any adverse? health effect, even in sensitive people.
These safe levels are usually based on information from experiments with animals (usually rodents) at much higher levels of the chemicals than humans would typically encounter. The higher animal exposures are used to see what the adverse health effects could be. The scientists then conjecture what the adverse effects possibly could be in humans at a lower level of exposure. Scientists can then estimate what a level probably is that will protect humans. Sometimes these safe levels differ among federal and state organizations because they assume different exposures, use different experimental animal studies, or employ methods that differ slightly. Sometimes the recommendations for safe levels differ because new science develops that suggests different levels are toxic or safe. EPA (2017) gives examples of these differing recommendations for 1,4-dioxane among government organizations. Recommendations and regulations are also updated periodically as more information becomes available.
Why Is EPA Looking At This Under The Lautenberg Chemical Safety Act?
Small excesses of the safe or virtually safe dose are not cause for concern since these safety levels are developed from conservative assumptions, including the use of safety factors that tend to exaggerate risk or exposure pathways that tend to exaggerate exposure. EPA scientists are currently looking at the likely routes of exposure to 1,4-dioxane in the environment because they want to develop exposure scenarios, or pathways, for the public.
These exposure pathways are being studied by EPA scientists by comparing the amount of 1,4-dioxane exposure in the pathway to its safe or virtually safe level. If exposure in the pathway is at or below this safe or virtually safe dose, then 1,4-dioxane exposure from the pathway is not a health concern. If exposure is above this safe or virtually safe level, then the pathway might be considered as a possible health concern; several pathways may be added together to suggest a health concern. In either event, regulations might be developed to lessen the exposure of 1,4-dioxane from this pathway.
Controversy Over 1,4-Dioxane
1,4-Dioxane has been shown to occur in foods by Nishimura et al. (2014)---up to 15 ppb in dairy products, dramatically higher than the virtually safe level developed by EPA (2013), that of 0.35 ppb in water, based on a non-threshold assessment of cancer effects in experimental animals. Although 1,4-dioxane causes cancer at high doses in experimental animals, EPA’s external peer review panel determined the safe level might actually be much higher and that a threshold assessment might be appropriate and outside investigators and the US National Toxicology Program explored this possibility. A paper was published in 2014 that supported the EPA panel’s threshold suggestion.
However, concerns still existed with one of the experimental animal studies, so the State of Kentucky petitioned the Alliance for Risk Assessment (ARA) to work collaboratively with four other US states and the government of Japan, to further explore this possibility and a second paper was published on this work. Health Canada is currently considering this collaborative ARA work in their assessment of 1,4-Dioxane. To settle the issue, EPA scientists are also considering this current work under the LCSA.
REFERENCES:
Dourson, M; Reichard, J; Nance, P; Burleigh-Flayer, H; Parker, A; Vincent, M; McConnell, EE; (2014). Mode of Action Analysis for Liver Tumors from Oral 1,4-Dioxane Exposures and Evidence-Based Dose Response Assessment. Reg. Toxicol. Pharmacol. 68(3):, 387–401. April 2014.
Dourson,M; Higginbotham, J; Crum,J: Burleigh-Flayer,H.; Nance, P; Forsberg,N; Lafranconi,M; Reichard,J. 2017. Update: Mode of action (MOA) for liver tumors induced by oral exposure to 1,4-dioxane. Regulatory Toxicology and Pharmacology. 88:45-55.
Nishimura et al., 2004. Study of 1,4-dioxane intake in the total diet using the market-basket method. Journal of Health Science. 50:101-107.
U.S. Agency for Toxic Substances and Disease Registry (ATSDR). 2012. Toxicological Profile For 1,4-Dioxane. April.
U.S. Environmental Protection Agency. 2013. Integrated Risk Information System (IRIS) for 1,4-Dioxane. National Center for Environmental Assessment. Washington D.C.
U.S. Environmental Protection Agency. 2017. Technical Fact Sheet – 1,4-Dioxane November 2017. EPA 505-F-17-011. Washington DC.
U.S. National Library of Medicine. ChemIDplus:dioxane Toxnet database found at https://chem.nlm.nih.gov/chemidplus/name/startswith/dioxane%20.
Authors:
Michael Dourson, PhD, DABT, FATS, FSRA
Bethany Hansen, MA.
Patricia McGinnis, PhD, DABT
All with Toxicology Excellence for Risk Assessment (TERA) of Cincinnati, Ohio, a 501c3 nonprofit organization created in 1995 with a mission to protect public health.


