
Hang on. This one is really unbelievable. The anti-opioid madness continues.
Normally I have a good deal of respect for FDA expert advisory panels (1). They consist of doctors and scientists (2) who take a serious look at both risks and benefits of a drug or device and make a recommendation to the FDA about whether they believe that the drug or device should be approved. The FDA can either accept or reject the panel's advice. The agency usually accepts the findings of the panel, but it is under no obligation to do so.
In general, I think they do a good job; most of the panels' decisions I have read are well thought out and logical. The risks and benefits of whatever is being evaluated usually make sense.
Not this time. Two different panels strongly recommended against the approval of another abuse-resistant form of oxycodone. The drug, which is called Remoxy ER, (Pain Therapeutics) got a 14-3 thumbs down by The Anesthetic and Analgesic Drug Products Advisory Committee and The Drug Safety and Risk Management Advisory Committee.
Most of the panel's reasons for rejecting the drug at least make sense, whether you agree with them or not (3). One example of the conclusion that the risk-benefit profile and safety of Remoxy ER was insufficient to support its approval was voiced by panel member John B. Hertig, PharmD, from the College of Pharmacy at Purdue University:
"I voted no because I think, ultimately, we need to do better for our patients both with chronic pain and those who struggle with abuse...I applaud the sponsor for being innovative and taking a step in the right direction but ultimately, when I'm balancing the risk-benefit and the availability of some similar options that are currently on the market compared with the possible public health impact, for me it was a no."
John B. Hertig, PharmD
Agree or not, this is a thoughtful and reasonable response. Hertig understood (and appreciated) the effort and technology that Pain Therapeutics applied to come up with Remoxy ER but didn't think that the new drug offered enough advantages compared to existing products. I cannot argue with his analysis, even though I question the harm of having another available option for pain patients who would benefit from an extended release oxycodone. This is when things get seriously screwy.
WHAT IS THE HARM? A ONE-WAY TICKET TO CRAZYVILLE
The reason I'm writing about this case at all is that one of the reasons given for rejecting the drug is SO stupid that it surprised even me, which is not so easy.
Remoxy ER was formulated as a viscous gel, which was designed to minimize abuse, which because of its thickness, is difficult to cut, divide and inject because it is so difficult to handle.
But during the presentation of the findings one FDA panelists noted that "while data met current standards for [abuse resistant] labeling via the intranasal route, the agency's lab showed that 'oxycodone suitable for IV [intravenous] use' could be extracted from the product under certain conditions."
This is just plain nuts.
Why is this nuts? Where to start?
- In an FDA chemistry lab, professionals found a way to make the stuff injectable. It probably took them months to figure it out.
- I have a bit of training myself and I don't have the wildest idea how they did it.
- So, is an addict desperate for a fix going to find out what the FDA chemists did in a fully equipped lab, take the pills to the men's room of the Port Authority Bus Terminal (4), repeat the procedure that was used, and then inject himself with oxycodone?
- NO! THEY ARE GOING TO SCORE SOME FENTANYL AND DROP DEAD FROM AN OD!
- Anyone with the intellectual capacity of a dung beetle should know this.
- Because the experiment has already been done. And failed.
- With a little chemistry, it was possible to turn abuse-resistant OxyContin into something that could be abused. Did this happen?
- No.
- But this sure did:
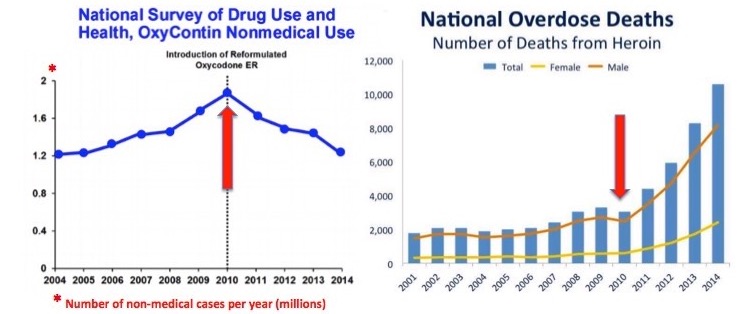
- When OxyContin was reformulated to make it abuse-resistant, addicts did not run out and get a Ph.D. in chemistry, and then after five years, figure out how to extract the oxycodone from the pill and then inject it. No, they stopped using it and ran to heroin instead. This is precisely when what we call the "opioid epidemic" started.
- How well did that work out?
- Of course, it's worse now. Heroin is now fentanyl. Fentanyl = death.
- Does anyone seriously believe that addicts are going to act any differently than they did in 2010 and start extracting oxycodone from Remoxy?
- No - they are not.
- Duh.
If the two panels really considered the minuscule possibility of Remoxy being converted to an abusable form of oxycodone and this played any part in their decision (and it sure sounds like it), then their credibility just went up in smoke.
How many times must our government run the same failed experiment before it figures out that it will only fail again? Nuts.
NOTES:
(1) I was nominated to be on an infectious disease expert panel only to be told that I "did not have the proper experience" to be on the panel. Which is kind of funny, since I spent half of my first career researching infectious diseases. Ah, government.
(2) Panels may also include consumer, industry, and patient representatives.
(3) In today's "opiate cleansing" climate, the term "makes sense" doesn't even make sense, since dogma, conformity, and hysteria have replaced critical thinking.
(4) For all you non-New Yorkers. For many years The Port Authority Bus Terminal, which opened in 1949, lived up to its reputation as one of the most disgusting and dangerous places in New York. In the 1990s "Operation Alternative" was put into motion and the terminal was renovated. It is now one of the newest most disgusting and dangerous places in New York.



