
No matter how clean it is, or how good the ventilation system, if you blindfold an organic chemist and walk him into a lab, he will know where he is 100 percent of the time. It's the smell. More precisely the combination of scents together. And if you've been playing with chemicals for long enough, you know that smells are usually not random. There are different odors associated with various classes of chemical classes. We call them functional groups.
Functional groups are critical to the study of organic chemistry because most chemicals within a functional group will behave similarly (and usually predictably) in chemical reactions. For example, a molecule that has a carbon-carbon double bond is called an alkene. Alkenes are known to react with hydrogen to form alkanes.
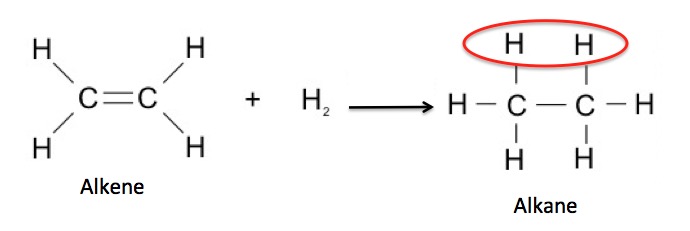
Reduction (addition of hydrogen) of an alkene to give an alkane. The red circle contains the two "new" hydrogen atoms
So, most (but not all) alkenes will behave in the same manner. This is how chemists choose what reactions are necessary to give them the desired product.
Alkenes (1) have another property in common—smell. Low molecular weight alkenes (2) have an unpleasant odor that is like gasoline or cleaning fluid. Here are a few common functional groups and their odors.
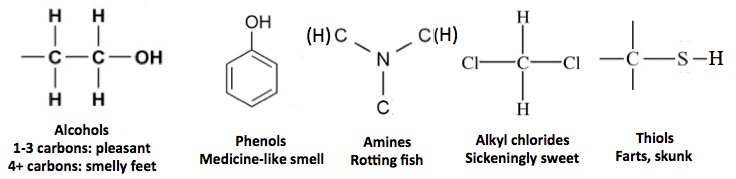
And here are a couple of others. Note the small difference molecular, but large olfactory difference when hydrogen is replaced with carbon in the molecule (red circles).
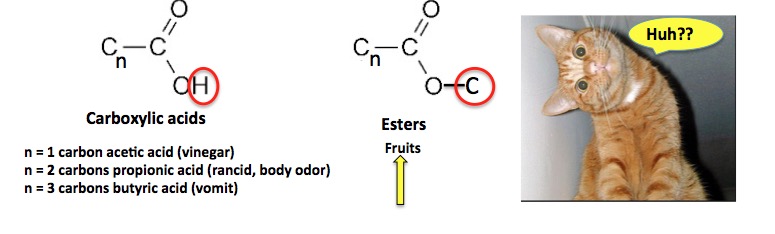
So, we magical chemists can take things that smell like body odor, smelly feet, and vomit and convert them to flavors and fragrances, simply because esters as a class, smell and taste good. Here are some examples (3).
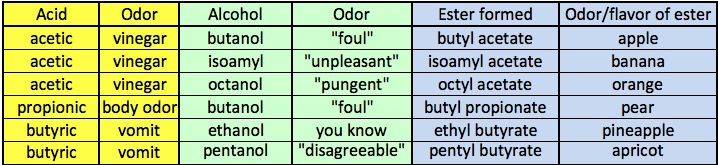
Some low molecular weight esters, their names and their odors.
So, in the unlikely event that you want to make your puke smell like pineapple, you can thank the chemistry gods for the Fischer esterification reaction. It is a very common method for forming esters from carboxylic acids plus alcohols.
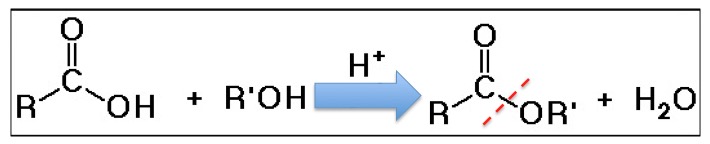
The Fischer esterification reaction (1895). A carboxylic acid is reacted with an alcohol to make an ester. The red line shows the new bond formed.
You now have all the information you need to turn your puke into pineapple oil, although this is not a commercially viable process. Put the puke into a round bottom flask, add ethanol plus a drop of sulfuric acid and heat it for a while. I won't bother how to tell you how to isolate the ethyl butyrate since this is probably the dumbest experiment ever conceived, but when the reaction is complete, it will smell like pineapple.
Perhaps a better source for pineapple would be the five pina coladas you consumed last night, which is what caused you to puke in the first place. Synthetic transcendental irony. Not something one gets to write about every day.

The late, great Warren Zevon (1978)
Notes:
(1) Another word for alkene is olefin
(2) Low molecular weight molecules are usually volatile, while the larger version of the same functional group may have no odor.
(3) The esters are almost always the same chemicals that give the fruit or flower its flavor or smell. When this is the case, the lab-made chemical and the one extracted from the fruit are indistinguishable.



