Fads come and go. And come and go again. (Repeat until comatose). So it should come as no surprise that we have another, especially one involving food, which is a superb spawning ground for fads, such as kale (1). It sounds rather stupid: Coffee with nitrogen bubbles. But it's quite popular. Perhaps enough so that it will get its own show on the Food Network—something that we non-cooking bachelors tend to avoid like a roaring case of salmonella. But does the chemistry hold up? Let's take a look.
Let's set preciousness of bubbly coffee aside for a moment. Is there any reason why it might make sense to actually put nitrogen in food or drinks. Possibly so, but first, you will need to sit through... the dreaded chemistry lesson.

Image: Pixabay
Elemental nitrogen, by far the most abundant component of our atmosphere, is a gas at atmospheric pressure and room temperature. But it is quite different than any of the other common gases that make up air. Chemically, it's dead boring because it's dead. Nitrogen is an inert gas, meaning that it won't react with anything (2). The only way that it can harm you (except for the bends) is if it is present in a sufficiently high concentration so that it displaces oxygen. Every now and then you'll read about industrial accidents in which workers died from nitrogen asphyxiation. Nitrogen can be insidious because it is odorless and gives no warning. In a matter of seconds, people pass out and never know what hit them. Death occurs shortly thereafter.
We experience no discomfort when inhaling nitrogen, which is very different from the awful gasping for breath that happens during suffocation. The gasping response is not due to a lack of oxygen; it is caused by your body's attempt to prevent the buildup of carbon dioxide, which will alter the pH of the blood - a big no-no. As long as this process is uninterrupted, carbon dioxide will not accumulate and it won't feel like you're being strangled. So, when you breathe in pure nitrogen, carbon dioxide continues to be expelled, so this reflex is not triggered. You just go to sleep and don't wake up. (3)
The inertness of nitrogen was at least one of the reasons that nitro-coffee was invented (4), and chemically, it makes sense for two primary reasons.
In 2013 Nate Armbrust, who would later become a food scientist (5), was contemplating what carbonated coffee might taste like. The answer: terrible. When carbon dioxide is dissolved in water a small amount of carbonic acid is formed. Acids have a sour taste (think: seltzer) and the concoction tasted awful. In order to get the fizziness without the flavor, Armbrust shuffled just a bit to the right on the periodic table - to nitrogen.
Since nitrogen is inert, it does not react with water and no acid is generated. So, the nitrogen-infused coffee tastes just like coffee. And nitrogen has another benefit - solubility in water, more specifically, its lack thereof. Here are the relative solubilities of nitrogen and carbon dioxide:
Solubility of N2 in water: 20 milligrams will dissolve in one liter of water. *
Solubility of CO2 in water: 1,750 milligrams will dissolve in one liter of water. *
* At 20º C and atmospheric pressure
Since nitrogen doesn't dissolve in water until it is pressurized, it forms very small bubbles, which do not change the taste of the coffee but do affect the texture. It becomes creamy, which some coffee drinkers seemed to love. The taste and texture spurred the marketing of the stuff by Stumptown Coffee Roasters.
In June 2013, the hipster coffee gourmands put it on tap at their Portland café. (5,6).
Chemical and Engineering News, August 24, 2015
Nitrogen provides another benefit, again because of its inertness. It displaces oxygen, which oxidizes (duh) some of the chemicals in coffee, and this prevents it from spoiling (7). But simply spritzing a little nitrogen in coffee isn't going to do much, since enough oxygen will still exist in solution to spoil the coffee. But the pressurized nitrogen also gets rid of the oxygen in solution- a process called degassing.
There about 1,500 chemicals in coffee. Table 1 shows what happens to four of the classes of coffee chemicals when they react with oxygen.
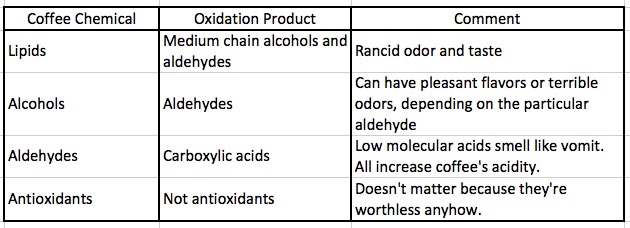
Table 1. Oxidation products of coffee and some of their properties
Lipids (oil, fats) react with oxygen to create medium-length chain alcohols. Unsaturated fats and oils (the so-called "healthy fats" - those that contain carbon-carbon double bonds) are more susceptible to degradation by oxygen than saturated fats (8), but both react to form a variety of alcohols and aldehydes. This process is called rancidity. Once formed, the alcohols that are formed react to give aldehydes. Low-to-medium molecular weight aldehydes can be mighty stinky. But not as much so as the carboxylic acids that are formed from the reaction of oxygen with the aldehydes. Low-to-medium molecular weight carboxylic acids have odors ranging from hideous to "you're gonna puke," which is fitting, since one of them, butyric acid, is what gives puke it's delightful bouquet.
Finally, coffee is full of everyone's darlings—antioxidants. Too bad antioxidants, which if you believe Crazy Joe Mercola, will make you live to be 175, have bombed in every clinical trial they've been in. The only use they have is for the stores that sell them. The five-dollar bottle of pomegranate juice may taste good but will protect you from cancer every bit as well as a one-dollar Mountain Dew.
So the use of nitrogen in coffee passes chemistry with an A grade. It does what it's supposed to do, and makes a cool product. Enjoy your nitro-coffee in the hipster cafe in Portland. Thanks to the nitrogen it will smell good. Probably much more so than the hipsters.
Notes:
(1) If kale is not the quintessential example of a fad food, then nothing is. Please give me any other reason why so many people are all of a sudden scarfing down this vile weed.
(2) Nitrogen does react with hydrogen and oxygen, but you really have to pound on it to makes this happen.
(3) For this reason, inhalation of nitrogen gas has become a popular method for committing suicide.
(4) This is not the first time nitrogen has been used in food. A recipe exists in a 1890 cookbook. Ice cream and beer are commonly prepared with liquid nitrogen.
(5) For the complete story of Armbrust's endeavors, see the 2015 article in Chemical and Engineering News.
(6) Hipster coffee gourmands in Portland cafes? Kill me.
(7) The inertness of nitrogen makes it indispensable in chemistry labs. Many chemicals react with moisture and/or oxygen in the air, so reaction flasks are flushed with nitrogen (or argon - another inert gas) and the experiment can be run without blowing your head off. (See: Butyllithium — Something You Really Don't Want To Mess With)
(8) This is why commercial bakeries prefer to use saturated fat (lard) than unsaturated fats- a longer shelf life. This is the same reason that trans-fats are/were used.




