With the catastrophic flooding that has devastated much of Houston, the very last thing the city needs is an additional problem. Especially a chemical explosion indirectly caused by the flood. But the city may be facing just that, and it's all because of the spontaneous decomposition of a class of compounds called organic peroxides (See below).
Organic peroxides are an unstable class of compounds which need to be kept cold. But power outages in the city have made refrigeration impossible in many places. One of these places is the Arkema chemical plant in Crosby, Texas. Arkema is one of the world's largest chemical manufacturers, and they make plenty of peroxides. Two explosions have already been reported, but they have been relatively mild - so far. Even so, residents of the area who live within 1.5 miles have been evacuated (1). But the real concern is what may happen if the plant catches fire. Assuming that significant amounts of organic peroxides remain in the plant, you do now want to be anywhere near there. Some officials have said that this is all but inevitable.
You do not want to mess with peroxides. As I wrote in my July 2016 article "Why Stuff Explodes," molecules that contain a disproportionate percentage of oxygen or nitrogen to carbon contain a very high amount of chemical energy within the molecule. It takes barely a nudge for this energy to be released in a violent explosion.
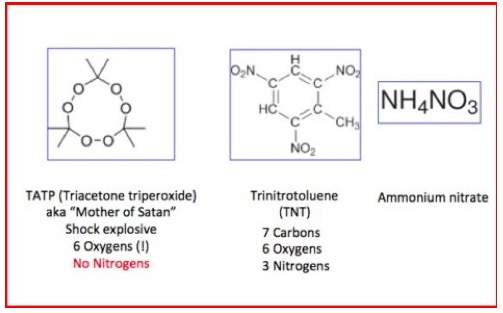
Examples of chemicals that contain a high amount of nitrogen and oxygen and a low amount of carbon. They are all explosives. TATP, aka acetone peroxide, is the explosive that Richard Reid carry in his should and tried to detonate it on a plane. Also, note the high proportion of oxygen and nitrogen atoms relative to carbon in each explosive.
Here is a historic example of what happened when ammonium nitrate, which contains only nitrogen and oxygen, can do. When the fertilizer caught fire in 1947 on a barge, the resulting explosion destroyed all of Texas City. About 500 people died and 4,000 were injured. It is highly unlikely that anything of this magnitude will be seen in Houston, but this disaster clearly illustrates the power of chemical explosives.

If things take a turn for the worse, you do not want to be in the area. Unsurprisingly, Arkema is issuing a note of concern:
'[I]f the volatile organic peroxides stored at the plant get too warm, some sort of explosion will happen...There is no way to prevent an explosion or fire."
Rich Rowe, CEO of Arkema's
Enough already. Houston needs a break. Let's hope for the best.
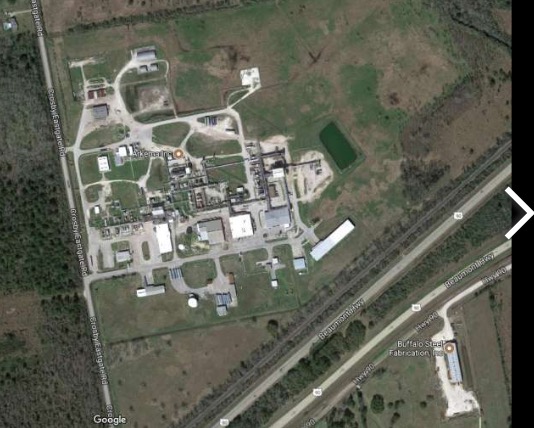
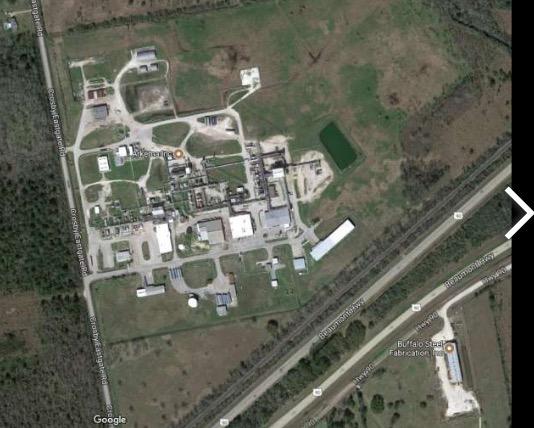
The Arkema chemical plant in Crosby, Texas. Photo: Google Maps
Note:
(1) The only "good" news here is that the decomposition products of organic peroxides are usually not toxic. Small comfort indeed.
(2) My thanks to Bob (comment below) for pointing out something that I thought was obvious, but may not be to everyone. Arkema is not making ammonium nitrate or TATP. These were merely examples of the explosive power of certain chemicals, which I added from an earlier article I wrote, strictly for educational purposes. The peroxide threat is real, but I had no intention of making this situation any scarier than it already is.



