The origin of life is a profound mystery. Once life arose, natural selection and evolution took over, but the question of how a mixture of various gases created life-giving molecules that arranged into structures capable of reproducing themselves remains unanswered.
Many theories have been proposed, some of which are popular (e.g., RNA World), and some of which are a far-fetched (e.g., aliens). Unlike politics, more ideas are not necessarily better; in science, a diversity of theories tends to betray the reality that scientists have no idea what's going on.
It is generally agreed that organic molecules were created when gases in the early Earth's atmosphere reacted. The trigger for these reactions is often attributed to lightning or meteorite impacts, but now a team of Israeli researchers has presented evidence that another widespread phenomenon could be responsible: Collapsing bubbles.
Bubbles are everywhere. Ocean waves, hydrothermal vents, rivers, and waterfalls create them. At the microscopic level, conditions inside collapsing bubbles can exhibit extreme temperatures and pressures of the sort necessary to trigger chemical reactions. Using this knowledge of what is called sonochemical synthesis, the authors created a computer model to determine what types of organic molecules could be synthesized inside collapsing bubbles.
Fire Burn and Caldron Bubble
The authors modeled 12 different initial conditions (represented by each box in the checkerboard figure), representing different combinations of gases: a carbon source (CO, CO2, or CH4) plus a nitrogen source (N2 with or without HCN or NH3 with or without HCN). After running the model, they authors "detected" (remember, this is a computer simulation) transient and stable reaction products.
(Note: The diagram below represents the model, with "cavitation" being the more formal term for "bubble collapse." The "shocked stage" section contains molecules that were detected transiently during the reaction, and the "cooling stage" section depicts stable reaction products.)
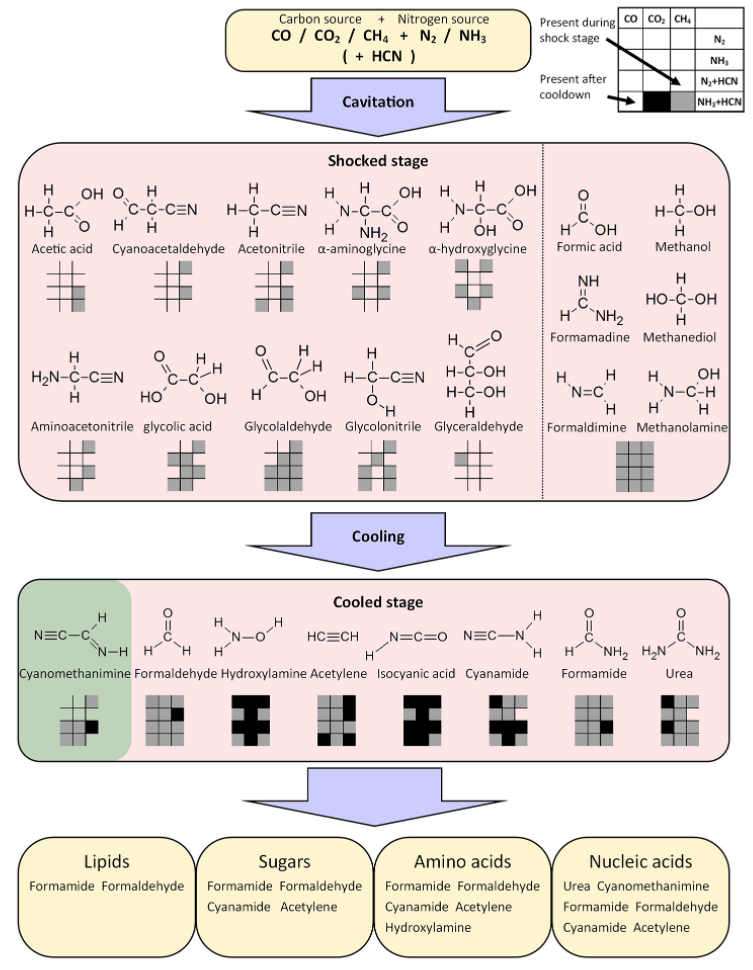
As shown, stable reaction products included many different biologically relevant molecules, which can go on to form things like lipids, sugars, amino acids, and nucleic acids.
Obviously, fancy models are just that: Fancy models. Without more solid data from chemistry experiments, their results are still highly theoretical. Still, the authors have provided insights into a mechanism that was proposed over 60 years ago but never seriously investigated. With time, perhaps the mystery of abiogenesis eventually will be solved.
Source: Natan-Haim Kalson, David Furman, and Yehuda Zeiri. ACS Cent. Sci. 3 (9): 1041-1049. Published: 11-Sept-2017. DOI: 10.1021/acscentsci.7b00325.




