Don't let the title discourage you even though it's pretty bad:
"Design of MC1R Selective γ-MSH Analogues with Canonical Amino Acids Leads to Potency and Pigmentation"
Yang Zhou, et. al., J. Med. Chem., 2017, 60 (22), pp 9320–9329 DOI: 10.1021/acs.jmedchem.7b01295 (University of Arizona Department of Chemistry and Biochemistry)
Most people brave enough to try to read this will probably understand approximately one word of the entire title: "with."
But if you can convince some sucker to wade through some pretty nasty biochemistry, there is a very cool in the story, and it will even explain what that demented title means. I am your designated sucker. This is the story. And it is fascinating.
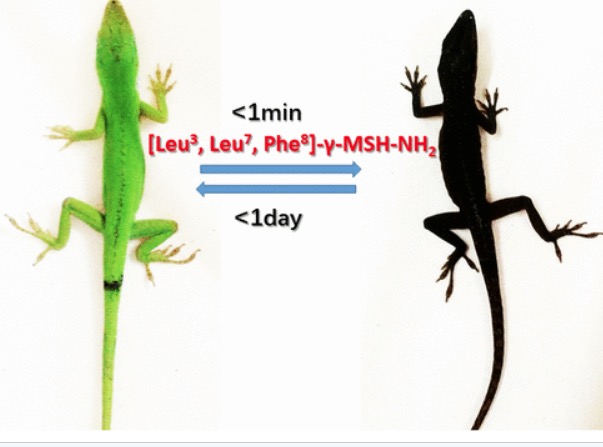
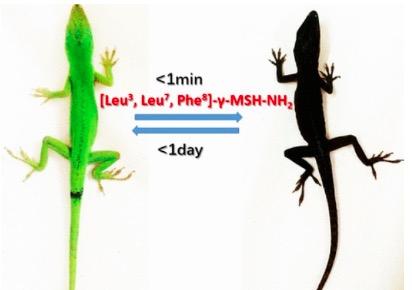
Figure 1. Good luck to the lizard census takers. "Excuse me, sir. What is your race?" Lizard: "Damned if I know." The black color is a result of increased melanin production.
No, what you see in Figure 1 is not a PowerPoint slide for a sensitivity training course. It's far more interesting (1). Make that fascinating. Instead, it is two photos of the same lizard taken 24 hours apart. When an experimental peptide (2) drug is given to a green lizard it turns black within one minute and stays black for about a day. Incredible. And also about as good a demonstration as you'll ever see of chemistry, biochemistry, and biology working together.

Amy Winehouse's" Back to Black" is not entirely accurate in this case, but the title "First Green then Black, then Back to Green" just didn't cut it. What a talent. A terrible loss.
The biochemistry is not only captivating, but the chemical reaction that makes this happen may have a very important medical application - a drug to prevent melanoma.
We are continually being bombarded by all sorts of radiation. Most of it is harmless, but UV radiation from the sun is not. Otherwise, 87,000 Americans would not be diagnosed with malignant melanoma each year, with almost 10,000 dying from it. Skin pigmentation is primarily melanin, which is a group of dark pigments (not a single molecule) found in the epidermis. Melanin "neutralizes" almost all of the sun's UV radiation (like a chemical umbrella) that hits our skin making it our natural defense against UV radiation, raising an intriguing question. If our bodies could produce more melanin would this translate into a lower incidence of melanoma? And how would you get the body to do this?
It turns out that it's not that difficult, at least in lizards. The production of melanin skin pigmentation is regulated by a receptor called MC1R (melanocortin 1 receptor). When MC1R is firing on all cylinders your body is a melanin-making machine. But is it possible to "encourage" MC1R to get working? Yes, it is. By exposing the MC1Rs to MC1R agonists - something that is shown quite clearly in Figure 1.
Mini biochemistry lesson time. Here is a very simple description of how receptors work (Figure 2):
- A receptor is a molecular switch that is embedded in cell membranes. Part of the switch is on the surface of the cell. The rest passes through the cell membrane into the cell and has a chemical switch on the inside of the cell.
- Certain chemicals fit the receptor binding sites on the outside of the cells.
- These can be agonists (results in an increase of some action inside the cell) or antagonists (results in a decrease in this action. (Figure 2)
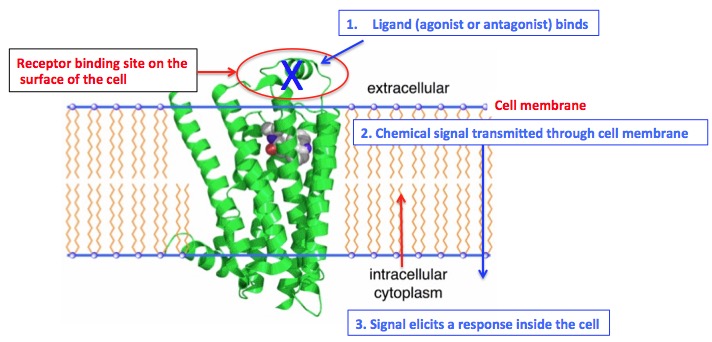
Figure 2: Simple cartoon of how a cellular receptor works. Original image: MoBio
The lizard experiment shows quite clearly that an MC1R agonist does what it's supposed - instruct MC1R to produce more melanin. It's black and green. Could this technology be used to protect humans from melanoma? Maybe. But as is usually the case with biochemistry, things are not simple - because there are multiple types of melanin receptors in the body, and setting these off can result in a host of adverse responses called off-target toxicity.
In fact, the same University of Arizona group previously discovered a drug called afamelanotide (brand name Scenesse) an MC1R agonist, which causes the body to produce more melanin. Scenesse is approved for a condition called erythropoietic protoporphyria, which causes sun damage that results in a painful, itchy rash, after even after a few minutes in the sun. People with erythropoietic protoporphyria cannot make enough melanin. But the off-target toxicity of Scenesse, which can cause headaches, nausea, loss of appetite, diarrhea, vomiting, migraines, dizziness... makes it unacceptable as a drug for the general public.
This brings us back to Selective γ-MSH Analogues with Canonical Amino Acids. (3) The key word is selective. The Arizona group has come up with different but related peptides, one of which is 16-times more potent toward hMC1R, a subtype of MC1R family of receptors, which the authors expect, when activated, will produce melanin without the side effects of the non-selective agonists.
Finally, we need to tackle the term "canonical," which involves a little lesson in peptides. Please do not hang yourself, as it will adversely impact my already-pitiful salary increase this year (4).
As I mentioned above, peptides are small chains of amino acids. All amino acids are given three-letter abbreviations. For example, Tyr means tyrosine, Val means valine, Leu means leucine, etc. The most promising compound in the paper, which the authors refer to as "Compound 5" has the structure:
Tyr-Val-Leu-Gly-His-Phe-Leu-Phe-Asp-Arg-Phe-Gly
Figure 2. The structure of "Compound 5," a 12 amino acid peptide. The amino acids to the right of Leu (bolded) are glycine, histidine, phenylalanine, leucine, phenylalanine, aspartic acid, arginine, phenylalanine, and glycine.
Now it is possible to explain what canonical means within the context of this paper. A canonical amino acid is one of the standard amino acids produced by our genetic codons. But look at the structure of afemalanotide. Nle is the abbreviation for norleucine, which is not one of the amino acids that form proteins and is, therefore, a non-canonical amino acid.

Figure 3. The structure of afamelanotide. Nle is the abbreviation for norleucine, which is not one of the 20 amino acids that form proteins. Thus, Nle is a non-canonical amino acid.
According to the authors, "The use of noncanonical amino acids in previous MC1R ligand development raises safety concerns." This may be related to the side effects of Scenesse. Or, the side effects could be due to its non-selectivity toward MC1Rs. Or both. Nonetheless, the possibility of a safe drug that could protect us from melanoma is intriguing. At the very worst you should now understand the title. More or less.
NOTES
(1) So is sorting lint
(2) Peptides and proteins are both made of chains of amino acids that are bound to each other. They differ in size (and also in function). Any two amino acids hooked together constitutes a peptide. The maximum number of amino acids is 50. After that, the molecule is designated as a protein. An average protein contains about 500 amino acids. The largest contains 27,000.
(3) γ-MSH is an abbreviation for γ-melanocyte-stimulating hormone. I suggest you ignore it.
(4) Who am I kidding? It's gonna be zero, just like last year, unless some of you guys out there write us a check.
Original source: J. Med. Chem. 2017, 60, 9320-9329, DOI: 10.1021/acs.jmedchem.7b01295