You have to be pretty much out of your mind to become an organic chemist. I base this not on hard data, but rather, on experience: a quarter of a decade being one as well as interacting with hordes of others.
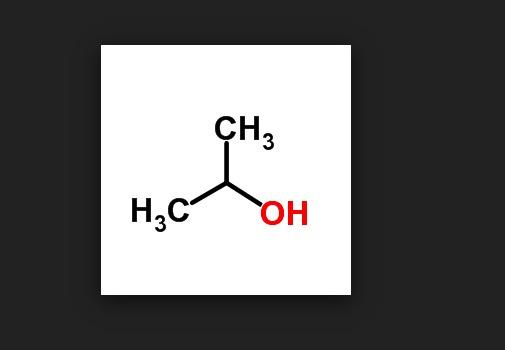
One of the (many) ways in which this lunacy manifests itself is the way we name molecules. And it would be hard to come up with a better example than a very common chemical that we have all used - isopropyl alcohol (rubbing alcohol). Seems simple enough, no? Actually, no. Here is a fine example of the madness. Ten different names for the same chemical.
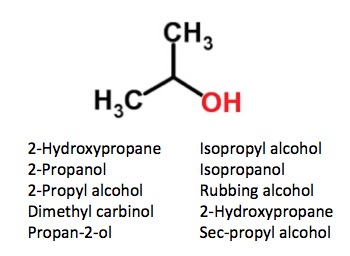
Ten names for isopropyl alcohol. Although some of these names are rarely used, they are all correct. And people wonder why organic chemistry is so stressful?
IPA (which is what most chemists call it, so you can now make that 11) is a very simple, small molecule, yet it has more names than episodes of Law and Order (and that's just counting English names). Which seems excessive by any measure. For some reason, this song comes to mind:

This song, which was performed by the group in America in 1971, won a Grammy. Pretty good song, but, I don't get the premise. The guy could have easily named his horse in the middle of the desert if he felt so strongly about its anonymity. It is unlikely that the horse would have objected. Original photo: Jukebox Time Machine
One of the reasons that organic chemicals (1) can have so many names is that many were named before the IUPAC (2) system was in use or because the IUPAC system, even though it specifically defines a unique chemical, is no picnic either (3). For example, here are two different names for a common chemical.
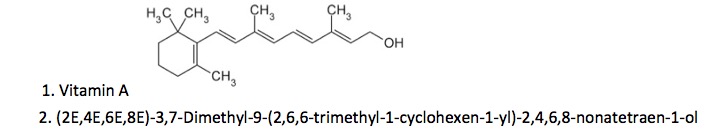
Both of these names are correct. Which would you choose?
Often, chemical names arise from the source of the chemical. Here are two examples, each with two names: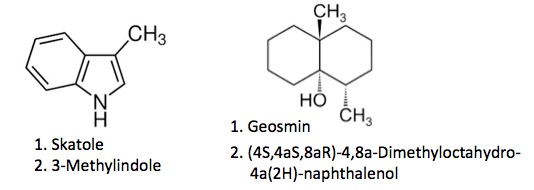
SKATOLE
Skatole (don't use this until you really have to, and even if you have to, still don't) got its name from skato, Greek for dung. If you open a bottle of the stuff you will quickly understand why (4). Skato also forms the basis for the word scatology. (If you don't know what this means look it up. My reputation is already bad enough).
GEOSMIN
Did you ever wonder why soil gives off a unique odor when it starts raining? It's because of a chemical called Geosmin, or (4S,4aS,8aR)-4,8a-Dimethyloctahydro-4a(2H)-naphthalenol if you prefer). Geosmin is produced in the soil by Streptomyces, a family of bacteria. The chemical stays in the soil until it gets wet or the earth is plowed, at which time minuscule amounts of it are released. People are capable of detecting very low concentrations of Geosmin. As with skatole, the name Geosmin's name is derived, not systematic.
As was the case with skatole, geosmin also gets its name from Greek, ge meaning "earth” and osmin meaning ”odor.” (Not surprisingly, geography was derived similarly, except "graphia" means description.)
PUTRESCINE AND CADAVERINE
But two chemicals that have really earned their names are putrescine and cadaverine, both bacterial breakdown of amino acids. These names sound like a joke. That is until you open the bottle. Then you will get a pretty good clue about where the names were derived: decaying flesh or meat. (5)
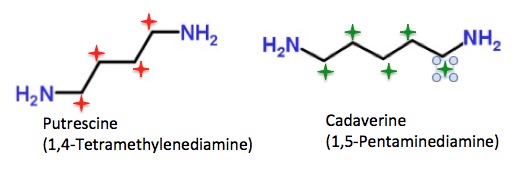
If you’ve never encountered them, the mere existence of compounds with names like putrescine and cadaverine should be warning enough.
Chemistry blogger Derek Lowe, "Amines and the Landscape of Chemical Stink"
Putrescine and cadaverine are almost chemically identical. The only difference is that putrescine has 4 carbon atoms (red stars) between the nitrogen atoms while cadaverine has 5 (green stars). Even though the two are very similar chemically (they differ by one CH2 (methylene) group and both stink to high heaven, they are biosynthesized from different amino acids.
And if the names putrescine and cadaverine are too subtle for you here are couple more polyamines: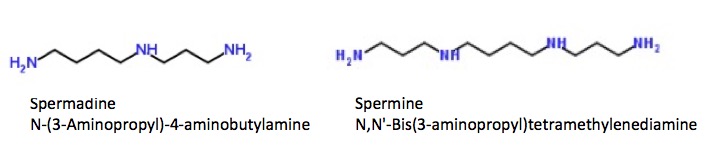
The chemical structures of spermidine (left) and spermine (right). Seriously??Tell me these weren't named by someone in the porn industry.
Before we quit, I thought it might be interesting to see some colorful descriptions of others who have encountered these chemicals:
"Diaminobutane and diaminopentane (cadaverine) are the worst. *Heaves*"
"One could also wear it when casually waiting out the zombie apocalypse so as not to attract the attention of a roaming horde of zombies." (My personal favorite)
And if music/poetry is your thing: I am only memories of lived deaths with a mind paralyzed by the bloody sunrise, to remember my past is to weep tears of cadaverine.
In case you thought that the New York City subway system was stinky.
NOTES:
(1) With very few exceptions, an organic chemical is a substance, man-made or naturally occurring, which contains the element carbon. See: ACSH Explains: What Does 'Organic' Really Mean?
(2) IUPAC stands for the International Union of Pure and Applied Chemistry. It was formed in 1919. Of all the chemists I know, approximately zero know how to use it for large, complex molecules. Not only is this an impossible task, but the software that generates a IUPAC name from a chemical structure can give different names (!) depending on which software was used.
(3) Most of the time IUPAC names are not seen on chemical bottle labels. The alternative way of assigning is name is called the "common system."
(4) Interestingly, in very low concentrations, skatole has a pleasant odor: It has been described like flowers, orange blossoms, jasmine. It is sometimes used in some perfumes.
(5) Cadaverine in low concentrations smells like semen. In fact, it is responsible for its odor. I'll leave the rest of that to Freud. Others say that it smells like rotting garbage. Rumor has it that kale eaters actually enjoy the scent.