
Since essential oils, such as lavender and tea tree oils have been all over the news for allegedly causing moobs, and I found that even my own colleagues at ACSH (1) didn't really know what the term "essential oil" meant I figured that it might be a good time for a chemist (aka dork) to step in and explain it. Don't feel bad if you're confused. Part of the problem is that English itself can be profoundly confusing. For example, try explaining to someone who is trying to learn English the pronunciation of these two states:

Original photo: Pinterest
Likewise, the word "essential" can have multiple meanings. one that is non-scientific, one that is nutritional, and one that is biochemical. I bet you don't get the last two right.
- Essential means "of the utmost importance."
- In the case of amino acids essential does not mean important. All 20 amino acids are required to make proteins), but only 9 are essential. This is because 11 amino acids are biosynthesized in your body and, therefore, not required in the diet. These are non-essential amino acids. The other 9 come from food. But don't let the names fool you; you would be seriously dead without all 20.
- Essential oils are neither essential or important. They just smell, which is the basis for their use in aromatherapy, perfume, food. The term is derived from essence: "an extract or concentrate obtained from a particular plant or other matter and used for flavoring or scent."
The reasons that different essential oils have different scents is because they are made up of varying amounts of a huge variety of plant-based chemicals called terpenes. Lavender oil is no exception; it is composed of more than 100 terpenes (2). Marijuana contains 200. Here are the four most prevalent terpenes in lavender oil (by weight):
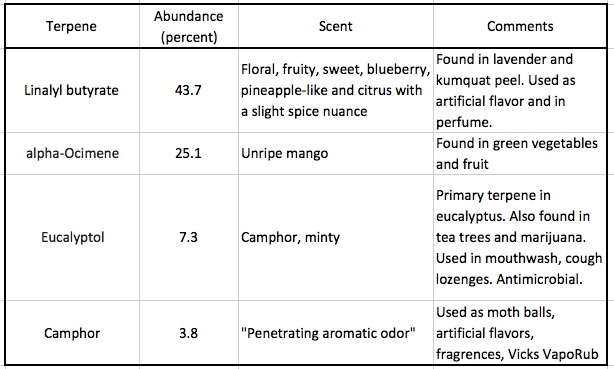
Terpenes are ubiquitous, naturally occurring chemicals, which are fascinating because even though there are thousands of them, they all share a single common structure called an isoprene unit, a five-atom collection of carbon atoms that always has the same framework (See Figure 1). All isoprene units bond to other isoprene subunits to form an enormous variety of terpenes of varying sizes and shapes, scents, and medicinal and toxicological properties. (Isoprene, a 5-carbon volatile hydrocarbon is given off in huge quantities by trees and plants.) Here are a few chemical structures of terpenes where you can (OK, might be able to) see how terpenes are all stitched together from multiple isoprene units (Figure 2). Good luck. You'll need it.
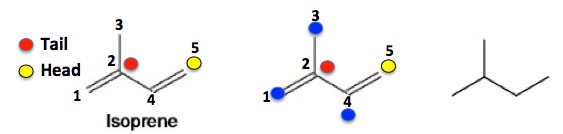
Figure 1. Isoprene is a five-carbon molecule which can be conceptually "broken" into two fragments (Left). the single carbon (yellow circle, #5) is called the "head" and the other three-carbon unit is the "tail "(red, carbons 1,2,3)." Note that the central carbon of the tail (red) is always connected to three other carbon atoms - 1, 3, 4 (blue) - while the head (yellow) is connected to only one (4, blue). This pattern is repeated over and over in nature, millions of times in the form of terpenes. Since all terpenes consist of the isoprene skeleton (image on right), which is not a real molecule, just its carbon skeleton, the components of terpenes are called isoprene units. But these are not always so easy to see. For example, vitamin A is constructed from four isoprene units (Figure 2).
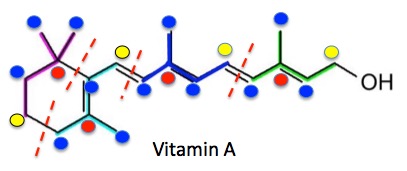
Figure 2. Vitamin A, a diterpene (3) consists of four isoprene units (purple, turquoise, blue, green) which are chemically attached to each other by four different bonds (red hatched lines). As is the case with isoprene itself, the head (red circle) is always attached to three other carbon atoms (blue circles) while the tail (yellow circle) is attached to only one. So, all isoprene units in Vitamin A (or any other terpene) consist of three carbon atoms with blue circles and two others with red and yellow circles. So, it should not be surprising that terpenes contain multiple units of 5 atoms- 10,15, 20 etc.
Finally, should you be bored with life or trying to cope with a disturbing amount of self-hatred, here is ß-carotene, a precursor of Vitamin A. Can you find all the isoprene units (Figure 3)? There are 8 of them. And please do not send me your therapy bills should you decide to try this. We are a non-profit. Not in the budget.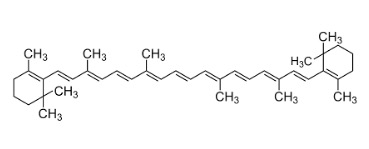
Figure 3. ß-Carotene contains 8 isoprene units. If you are completely out of your mind, feel free to try to find them.
And if you are "Son of Sam Crazy" try cholesterol (Figure 4). It isn't technically a terpene because it has only 27 carbons (4), but is sort of is anyhow because it is biosynthesized from lanosterol, which is. Later on, three carbon atoms are chopped off. Can you identify the 6 isoprene units in lanosterol and then figure out which three carbons are lost in the conversion to cholesterol? I could, but I'd rather be shot, hung, and then set on fire.
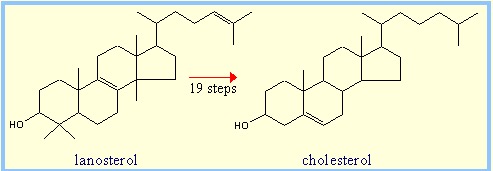
Figure 4. The bioconversion of lanosterol, a terpene which contains 6 isoprene units, to cholesterol.
NOTES:
(1) Some of us here have moobs. Some don't. That is all I shall say.
(2) Lavender oil is nothing more than a bunch of terpene chemicals. So is turpentine (paint remover). Your choice.
(3) The smallest terpene (with the exception of isoprene itself) consists of two isoprene units, so it contains 10 carbon atoms. Larger terpenes have different (and strange) other names. Here are a few more:
- Monoterpenes have 10 carbons. (Molecular formula C10H16). Very common
- Sesquiterpenes have 15 carbons. (Molecular formula C15H24)
- Diterpenes have 20 carbons. (Molecular formula C20H32). Very common
- Sesterterpenes have 25 carbons. (Molecular formula C25H40). Very uncommon. Try using this one in Scrabble.
(4) For this reason, cholesterol is considered to be a terpenoid.



