I recently wrote about three of the deadly neurotoxins being produced by cyanobacteria (aka blue-green algae) during an ongoing algae bloom in South Florida (See Florida's Deadly Algae Bloom - Why Is It So Dangerous?). The toxins range from structurally simple and easy for organic chemists to synthesize in the lab to moderately complex and not simple at all.
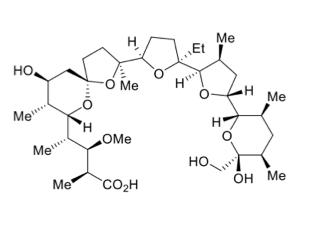
But there are numerous examples of plants, marine organisms, and bacteria that easily biosynthesize molecules that are so complex and difficult to make synthetically that chemistry grad students and post-docs who were given the unenviable task of doing so are probably still waking up in cold sweats thinking about what they went through decades earlier. Pity the unfortunate souls who were given the unenviable task of trying to synthesize monensin (Figure 1), an insanely complex ionophore antibiotic (1) that was first isolated in 1967 from Streptomyces cinnamonensis, but not synthesized in the lab until 12 years later (2,3,4).
Figure 1. (Left) The chemical structure of monensin - a chemists' worst nightmare. (Right) One of the hundreds of post-docs who gave up any semblance of a life to complete the total synthesis of the compound. Little-known factoid: there was a loophole in the Emancipation Proclamation which excluded post-docs.
The details (Figure 2) of the first total synthesis of monensin demonstrate why the field of total synthesis is typically useful for training synthetic organic chemists to "screw molecules together" but not much else. 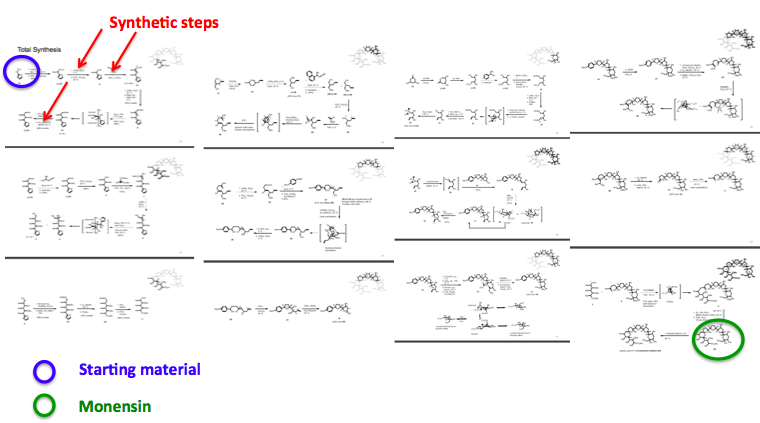
Figure 2. A very condensed view of the synthetic scheme used by Yoshito Kishi's group at Harvard in the first total synthesis of monensin. Or a depiction of Dante's Vestibule of Hell. Each red arrow represents a "step," aka a synthetic transformation. There are 73 of them. Source: msu.edu
A little more about synthetic transformations. These are just what the name implies - the conversion of one molecule into another by using a reagent (a chemical or catalyst that promotes a chemical reaction). Figure 3 shows a very simple example (5) of a synthetic transformation.
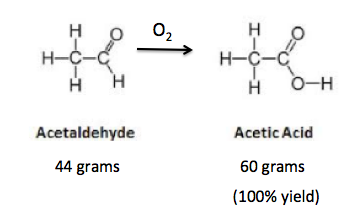
Figure 3. The reaction of acetaldehyde with oxygen to form acetic acid.
Acetaldehyde can be easily converted to acetic acid by a wide variety of oxidizing agents. I use oxygen in this example. If the reaction is perfect you can expect 60 grams of acetic acid to be formed (6) from 44 grams of acetaldehyde. But no organic reaction is perfect. You almost never get 100% of the amount of the reaction product you expect because byproducts (impurities) are formed in almost all chemical reactions, especially those involving complex structures. These impurities decrease the amount of the reaction product by some percent. The amount of product obtained compared to the amount expected (in a perfect reaction) is called the synthetic yield. When the synthetic yield is below 100% some material is almost always lost in every synthetic step. The more steps, the more material gets lost during the sequence. These losses add up fast.
A very clean reaction will give a 90 percent yield, with the rest being impurities. This is excellent (and not so common), but it illustrates the amount of material that is lost in even a moderately short (10-step) reaction sequence with each step having a 90% yield.: (0.90)10 = 0.35. In other words, the overall yield is only 35%; you lose 65% of your material along the way (7).
But a more realistic (and still very good) yield for an organic transformation is 80%. Doing the math, a 10-step reaction sequence with each step yielding 80% will give you an overall yield of 11%. But it's even worse. Some reactions are just filthy messes. Organic chemists REALLY hate the 20% yield reactions, but they happen. The same sequence with nine 80% yield steps and one with a 20% yield gives an overall yield of 3%. It becomes evident that with long synthetic sequences that you're going to have to start with a whole lot of material in the first step to get much at the end. And the Kishi total synthesis is a whole lot worse than this (Figure 4).
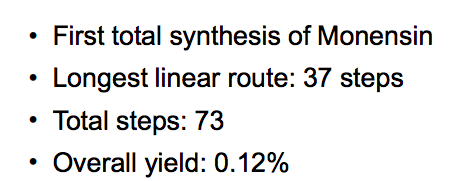
Figure 4. Summary of the first total synthesis of monensin
An overall yield of 0.12% means that to get one gram on monensin you have to begin with 83,333 grams of starting material (and 83,334 post-docs). Yoshito Kishi is considered to be one of the premier synthetic organic chemists of his time and his total synthesis of monensin was a historic milestone in the field of total synthesis. But it took 12 years from the isolation of monensin (and the sanity of countless post-docs) to make something that single-celled microorganisms can do in a few minutes. Ain't nature grand?
NOTES:
(1) Ionophore antibiotics were originally used to treat intestinal parasites in livestock are now also used as growth promoters. They work by increasing the flow of metal ions through bacterial membranes.
(2) The art of total synthesis is usually no more than an intellectual exercise in which heads of big academic research groups compete to be the first to make a particular molecule from scratch. The molecule they pick is of interest because of its structural complexity, interesting biological activity, or both. It is rare when the synthesis has much utility; the living organism does the job much better.
(3) Semi-synthesis, in which the starting material is isolated from the plant (etc.) is much more practical than total synthesis. Perhaps the prime example is the semi-synthesis of the cancer drug taxol. Prior to the semi-synthesis of taxol, the drug could only be obtained by stripping the bark off the Pacific Yew tree, which killed the tree. To provide enough taxol to treat ovarian cancer in the US 360,000 trees would have to be killed. Then Robert Holton a professor of chemistry at Florida State University figured out how to make the drug very easily. He licensed the process to Bristol-Myers Squibb for a cool $351 million. (See: Semisynthetic: A *Real* Word That Saves Lives.)
(4) There is plenty of ego involved as well. The group that gets there first gets the "glory," but credit can also go to a group that comes up with a more efficient or shorter synthesis. There is an infinite number of ways to make these complex molecules, some better than others.
(5) But one of significant biological importance. In the body, alcohol is metabolized in two distinct steps to give acetic acid (vinegar). The first step is the conversion of alcohol to acetaldehyde, which is quite toxic and responsible for your hangover. The second step converts acetaldehyde to harmless acetic acid. In the absence of the second step, you will become violently ill. (See Antabuse - A Very Good Pill To Stop Drinking, But Don't Cheat)
(6) If you don't know why don't ask. It's called stoichiometry and will make your head explode.
(7) This assumes no change in molecular weight from the starting material to the final product.



