Plastic pollution of our oceans, especially in Asia (See Compostable Forks Don't Work And Paper Straws Are Nasty. Thanks, Seattle! by my colleague Dr. Alex Berezow), is a real problem without a real solution. As I have written here and here, there are some very clever chemists out there trying to tackle the problem but no existing method seems robust enough to be technically and/or economically feasible on a large scale.
With biodegradable plastics, it is the rate of decomposition and the chemical products of degradation that play a part in determining the feasibility of a given method, especially on a large scale; the plastic must break down quickly and give non-toxic byproducts for a method to be useful. Two plastics that do not meet these qualifications are polystyrene and vinyl (polyvinyl chloride, PVC). Polystyrene, when used as Styrofoam, is mostly air and is very difficult (and not economically feasible) to collect. PVC is biochemically stable (although there are ongoing efforts to identify bacteria that will degrade it).
A look at the chemical structures of the monomers of polystyrene and PVC reveals a common structural element - a carbon-carbon double bond (red oval). Molecules that contain these are called olefins or alkenes (Figure 1).
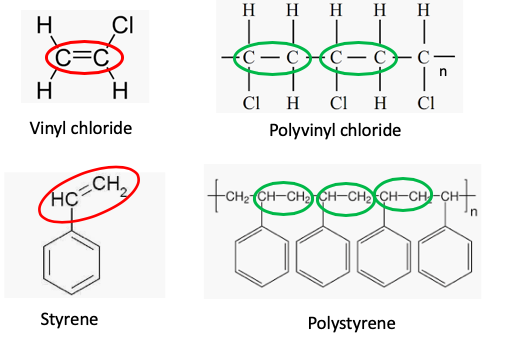
Figure 1. The chemical structures of PVC (top) and polystyrene (top). Olefins (double bonds are shown in red ovals) can be converted to long chains of repeating units (green ovals) by a simple chemical reaction called polymerization. The letter "n" represents the number of repeating units in the polymer (usually thousands). (1)
A very interesting concept that makes use of olefin polymerization in an environmentally friendly way was just published in the journal Polymer Chemistry. Researchers at the Research Initiative for Supra-Materials at the Shinshu University in Nagano Prefecture, Japan were interested in finding a plastic that could be made from and degrade to a natural product, which could then be recovered and recycled. They chose aspirin - a simple derivative of salicylic acid. Salicylic acid is found in the bark of willow trees. Prior to aspirin, it was used to relieve pain and reduce fever. It is not possible to make an olefin from salicylic acid, but, thanks to some clever chemistry developed by the Shinshu group, it is possible to do so from aspirin (acetylsalicylic acid, ASA). Here's the chemistry: (for dorks only, the rest of you can leave now, Figure 2).
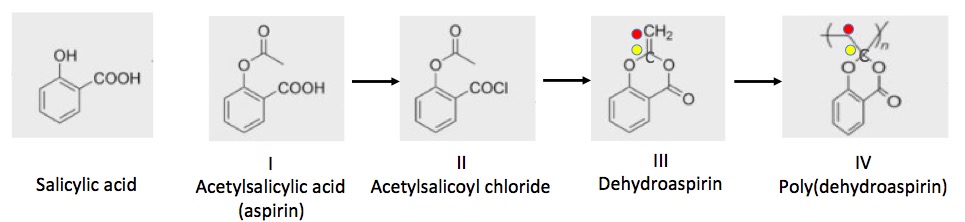
Figure 2. (1) Aspirin (I), which is made from salicylic acid (Left), is converted to acetylsalicoyl chloride (II) using thionyl chloride - a simple reagent that is known to carry out this reaction. (2) The acetylsalicoyl chloride (II) is then reacted with triethylamine, a very commonly used base, which converts it to dehydroaspirin (III). Dehydroaspirin is an olefin and thus capable of polymerization to form "aspirin plastic" (IV). The three reaction sequence consists of well-known and efficient synthetic steps. For perspective, the yellow and red dots have been added to show the olefin portion of the molecule in III and the corresponding two carbon atoms in the polymer IV.
But here is the best part (Figure 3)...
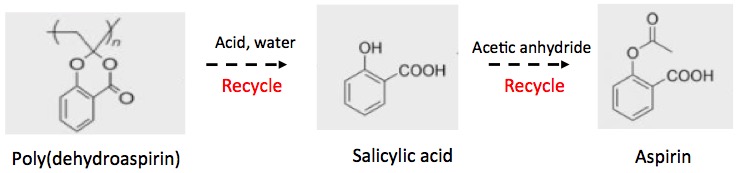
Figure 3. The poly(dehydroaspirin) ( "aspirin plastic") need not be degraded with bacteria, which can be slow and temperamental. Instead, it can be cooked up with a mineral acid (e.g., hydrochloric or sulfuric) and water - a 5,000-year old reaction called hydrolysis (2). The hydrolysis reaction breaks apart the polymer, giving back salicylic acid - the original chemical substrate, which can be recovered and then reacted with acetic anhydride - another common reagent - to make aspirin. This is the same method that Bayer used in 1897.
The Shinshu group is experimenting with different conditions to improve the properties of the "aspirin plastic," but the process they have developed is elegant and simple; it starts with aspirin and ends up with aspirin. Which could mean less pain for the environment. We wish them well.
NOTE:
(1) Although styrene and vinyl chloride are both carcinogens their polymers are not. The corresponding polymers share only the name of the monomer from which they were derived, not the properties. Scare groups like NRDC and EWG take advantage of the similarity in the names to imply that the polymer plastics are also carcinogens. As usual, when these groups talk about chemistry they are either lying, ignorant or both.
(2) The conversion of fat to soap was discovered by the ancient Babylonians around 2800 BC. This is a hydrolysis reaction.
Source: Akane Kazamaa and Yasuhiro Kohsaka. "Radical polymerization of ‘dehydroaspirin’ with the formation of a hemiacetal ester skeleton: a hint for recyclable vinyl polymers." Polym. Chem., 2019,10, 2764-2768. Published: 7-May-2019. DOI: 10.1039/C9PY00474B




