Here is what the average American knows about gold:
- It's pretty
- It costs a lot...
- (For guys only) ...but not as much as alimony, should you forget to purchase a sufficient quantity of said gold to commemorate various occasions of importance, especially anniversaries.
This statement can be represented by a mathematical equation:
Credits: Divorced Girl Smiling, Alibaba
But there's plenty that you don't know about gold, for example, you can chew it (sometimes), you can destroy it (but it's not easy), there are a few (terrible) drugs that contain it, and miners use sodium cyanide to extract it, which isn't exactly marvelous for the environment. Before we get to the Chemistry Lesson From Hell,® a little about gold "minerals."
I put the word minerals in quotes because gold (and seven other metals) is found in the earth in its native state – metallic gold. This is because it and the other noble metals – those that are stable to oxygen, carbon dioxide and all the other stuff in the environment – are chemically unreactive (mostly). So, while all the magnesium, sodium, potassium, zinc, lithium, many others... on earth are found in minerals as chemical compounds of the metal (for example, calcium is often found as calcium carbonate, chalk) gold is chemically unchanged and is found as gold metal.
There is an exception. Gold combines with tellurium (of all things) to form gold telluride, a mineral. This is seriously weird chemistry so rather than make an idiot out of myself trying to explain it I'll just show some pretty pictures.

Gold tellurides. Photo: 911 Metalurgist
OK, let's get on with it...

Things that you probably don't want to know about gold but you will read anyhow, if only to placate me:
- Gold is practically inert
There are very few things on earth that won't react with nitric acid. It's some powerful ####. But gold does not. You need something even worse called aqua regia ("regal water"), which is made by adding 3 parts of concentrated hydrochloric acid to one part concentrated nitric acid. (Warning - not recommended as eye drops). The reason both acids are required is that they perform different functions in two distinct steps. Nitric acid is a very strong acid but also a powerful oxidizing reagent. Hydrochloric acid provides Cl-.
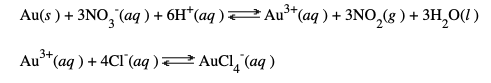
The reaction of gold metal with aqua regia. Gold reacts reversibly (and very slowly) with nitric acid to form a little Au3+. Then chloride reacts with the Au3+ to form the stable AuCl4- ion, which is water-soluble. This reaction removes the Au3+ from the solution and drives the equilibrium to complete the reaction.
- Pure gold is too soft to make jewelry, so much so that you can chew it.
There are a number of methods used to measure the hardness or softness of metals. (One is called the Rockwell Hardness Scale.) By any measure gold (24K, 100% pure) is soft. It is rarely used in jewelry because it would bend too easily. There is another measure of metallic hardness called the Mohs Scale, which rates metals from 1-10 with 1 being the softest (1). Gold, zinc, tin, and lead (the softest of the common metals) are on the low end (between 1.5-2.5) while steel and tungsten are on the other end (~7). Your teeth are a 5, so while it is not recommended, if you bite down on pure gold you will leave a small imprint. If the gold is thin enough it is possible (but not especially wise) to chew it.
- Gold goes well with fries. Maybe.
If you're a real moron, especially a wealthy one, you can "eat" gold. A food truck (in New York, of course) was selling a $666 Douche Burger, which was Kobe beef and a bunch of other icky stuff wrapped in gold leaf. The owner, Franz Aliquo, had his reasons:
"[We make it because of] our deep-seated disgust and hatred of all the other douche burgers out there."
Franz Aliquo, 2012. One angry dude, but the quote is pure gold.
It turned out to be a hoax.

Image: YouTube
A Douche Burger. You are what you eat. Photo: Mackenzie Keegan, Time Magazine
4. Gold forms coordination complexes. Some of them are drugs.
Gold doesn't react with many chemicals, but it is a transition metal – a group of metals in the center of the periodic table that form "coordination complexes" with the metal in the center surrounded by a number (often 4 or 6) of ligands (2) such as ammonia, cyanide, water, and chloride. These metal-ligand bonds can be quite strong, even irreversible. To envision the difference between a chemical reaction and complexation, a reaction "destroys" the metal while coordination "holds it captive." Here's a notorious coordination complex:
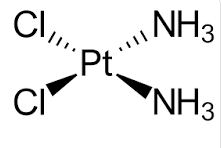
cis-Diamminedichloridoplatinum(II), aka cisplatin, is a coordination complex with two chloride and two ammonia ligands. It is one of the most toxic chemo drugs, causing vomiting in >90% of patients.
If you haven't done research in the arthritis field you might be very surprised to know that there are actually FDA approved drugs containing gold atoms. Gold-containing drugs are immunosuppressive coordination complexes (with sulfur) and are used to treat rheumatoid arthritis. Here are their structures.
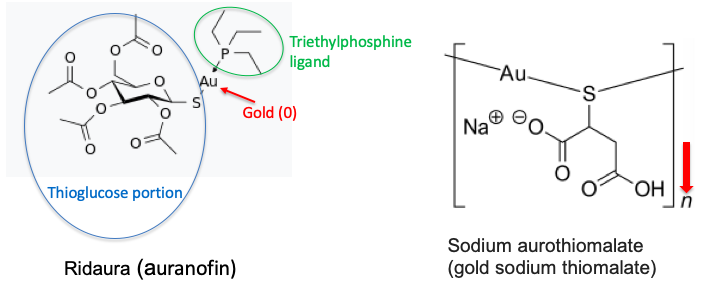
Two gold-containing drugs. Both Ridaura (a complex of acetylated thioglucose and triethylphosphine) and gold thiomalate (a polymeric complex of gold and thiomalic acid) are disease-modifying anti-rheumatic drugs (DMARDs). Both drugs have a ton of side effects and don't work very well. They have mostly been replaced by newer biological immunosuppressants like Enbrel and Humira.
- Gold mining isn't so great for the environment
Gold metal also forms coordination complexes with cyanide, which is why cyanide is used for gold mining. In a process called cyanide leaching, crude gold-containing ore is soaked in a solution of sodium cyanide.
Step 1: Gold is reacted with sodium cyanide and oxygen to form sodium dicyanoaurate (complex), which is water-soluble (this is what "aq" means):
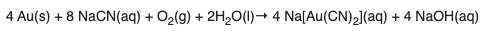
Step 2: The gold complex is reacted with powdered zinc, forming zinc cyanide (water-soluble) and gold metal, which precipitates and can be collected by filtration.
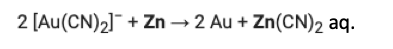
Problem is that this can leave a bunch of cyanide lying around, which is almost as bad as kale. The US has banned the use of cyanide leaching to mine gold. It has yet to ban kale.
Well, time is money and money is gold. I've spent more than enough time writing this hideous article. Feel free to symbolically balance the equation by sending some gold to ACSH. We won't waste it on $666 hamburgers.
NOTES:
(1) The alkali earth (Group 1) metals are all softer than lead. For example, potassium is 0.4. But if you bite a piece of potassium it will blow your head off. And the guy next to you.
(2) In chemistry, a ligand is an ion or molecule attached to a metal. In biochemistry, it is a small fragment attached to a larger one.




