The basis of a new, non-toxic, recyclable, fireproof insulation foam is high school chemistry. Maybe even middle school. But that doesn't make the invention of a calcium carbonate (chalk)-based product any less clever or useful. It's both.
There are a wide variety of materials used to make spray foam insulation, ranging from highly flame-resistant to highly flammable to toxic. Polyurethane foams are both flammable and give off toxic gases when burned. They are also made from a class of compounds called isocyanates, which are notoriously toxic.
Now, research conducted at the University of Konstanz and the University of Stuttgart, which was recently featured in Chemistry World, examined a "foaming mineral plastic" that has a number of desirable properties. It is made from simple materials, fireproof, and can be recycled by making use of the simplest of chemical reactions. An added bonus – when you try to burn it the foam generates carbon dioxide – the chemical in fire extinguishers –to put itself out. Very clever, indeed.
The ingredients of the mineral-based foam are calcium chloride, ammonia, carbon dioxide, and polyacrylic acid, which is safe enough to use in eye drops. All of them can be purchased at a supermarket.
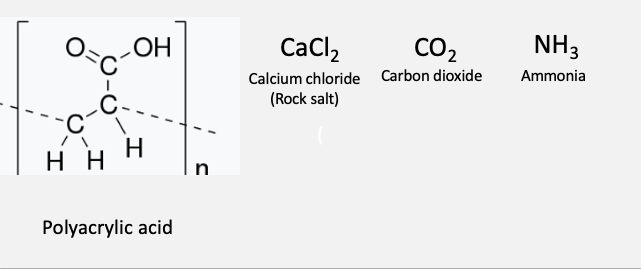
The simple chemicals used to make fireproof mineral foam.
Here's how the foam is made. The chemistry is simple.
Polyacrylic acid, a widely-used, non-toxic polymer of acrylic acid is mixed with calcium chloride and a non-ionic surfactant (common chemicals that are used as soaps, detergents, and emulsifying agents). Then the mixture is stirred to allow air to infiltrate the foam. At this point, the foam mixture is acidic, so the team (this is simple but really clever) added ammonia, which makes it basic. Now it's high school chemistry time – simple acid-base chemistry.
Ca2+ solution (calcium chloride in water) + base --------> basic Ca2+ solution
Basic Ca2+ solution + carbon dioxide (from air) ---------> calcium carbonate
The combination of calcium carbonate and polyacrylic acid and air is now suitable to be used as an insulating foam.
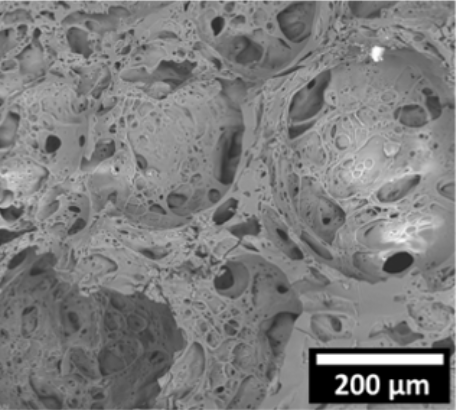
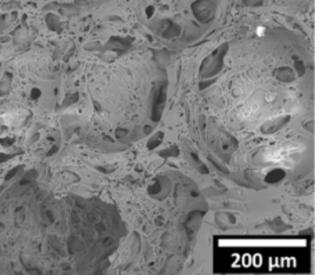
Scanning electron micrograph image of the mineral foam. 200 µM is 0.007 inches. Credit: Cosima Stubenrauch/Universität Stuttgart
But, it gets even better. When heated, the polyacrylic acid doesn't burn; it just decomposes and turns black. And something else happens:
CaCO3 + Heat -------> CaO (calcium oxide) + CO2

Not only does the foam not burn, but when heated the calcium carbonate decomposes, giving off carbon dioxide in the process – little molecular fire extinguishers. How great is this?
But, we're not quite done with the chemistry (apologies). When it's time to get rid of the insulation it doesn't need to be thrown out. Just chuck it in with hydrochloric acid...
CaCO3 + HCl (aq) -------> CaCl2 + CO2
... and the calcium chloride, which was one of the starting materials, can be recovered and used again. How? It's the same reaction we started – the reaction of calcium chloride with carbon dioxide.

The recycled solution contains calcium chloride, the polyacrylic acid, and whatever insoluble crap happens to be floating around in there. The crap can be removed by filtration, which leaves a clear solution. Then carbon dioxide is added and boom! - a white solid precipitates (the down arrow in the equation), which can be collected by filtration. Now, the calcium has been recovered.

Calcium carbonate precipitating in a test tube. Credit: ChemSkills
It would be difficult to come up with a better example of how simple chemistry when applied in the right way, can solve real-world problems. Hats off to Cosima Stubenrauch (University of Stuttgart) and Helmut Cölfen (University of Stuttgart) for this simple, but elegant solution (2).
‘They are not the first to have thought about it, but they were the first to actually implement it and I like it very much. Although the road to practical application of these foams in building insulation is still long, we can now at least imagine what the material of the future is in this field.’
Sebastijan Kovačič, a polymer scientist at the National Institute of Chemistry, Slovenia
NOTE:
(1) Drs. Stubenrauch and Cölfen have also published their work in the journal Materials Horizons.



