In the mood to read about an element this morning? Probably not. But since you know this is going to be incredibly fascinating, mind-altering, and most likely in poor taste, thousands of you are going to read it anyhow (1). Don't fight it.
Iodine is an unusual and fascinating element. Some of its functions include:
- Antiseptic
- Cloud seeding
- Protection against radiation poisoning
- Blowing stuff up
Iodine itself is not found in nature
Elemental iodine, also called molecular iodine (2) is too reactive to be found in nature. But it does exist in spectacular minerals, the most common is called iodagyrite, which is composed of silver iodide. This is not a coincidence. More on that later.

(Left) Elemental iodine (Middle, Right) Iodagyrite (Silver iodide) Photo credits: Stephen Wolfshreid, Mindat.org
Iodine is a halogen
Near the right side of the periodic table (not political) is a column containing Group 7 elements, aka, the halogens. You've probably heard of all of them: fluorine, chlorine, bromine, and iodine. But...
Intruder Alert!
...the halogen group has two unwelcome intruders: astitine and tennessine. Based on my years of experience as a chemist, I can offer the following in-depth analysis: they are stupid.
Stupid elements!
Photo credit: Flickr
I've been a chemist for 95 years and had to look up At (astatine) and Ts (tennessine) on Wikipedia just to learn their names. I knew nothing about them, which is fine since they are essentially "non-elements." They don't belong on any table, let alone the periodic table (just my opinion; sorry, physicists). Here's why:
Astatine is highly unstable and radioactive, so it is constantly decaying. Some more useless factoids:
- At any given time, a grand total of one gram exists on Earth, making it the rarest element in the Earth's crust.
- If you managed to get enough to put it in a bottle, it would vaporize itself and the bottle because of the heat generated by radioactive decay. So, no one knows what it looks like.
Tennessine is even worse. It doesn't even exist. It was "discovered" in 2009 when scientists blasted something or other with something else and detected it with god-knows-what (3). A grand total of six atoms was made. I say get this s### off my periodic table!
Do these things belong anywhere on the periodic table, let alone in the halogen group? Judges?

Photo: Pixabay
Iodine is an antiseptic
In the halogen group, the elements become less reactive going from top to bottom. Fluorine, which is the most reactive of all elements, is simply terrifying (See Fluorine, the Element From Hell). Chlorine, which is not nearly as bad as fluorine, is bad enough to be one of the worst chemical weapons. It was used in World War I. The results were horrendous.
If you're expecting bromine, a dark red liquid at room temperature, to be a walk in the park, think again. Its fumes are toxic as hell. I've used it exactly once. Some of the fumes splooted out of the bottle and landed on my arm. That felt like an oxygen-acetylene torch. It's not until you get down to iodine that things get calm enough to make it easy to handle. It is used as a topical antiseptic agent. A complex of iodine and polyvinylpyrrolidone (aka povidone) and iodine called polyvinylpyrrolidonepovidone-iodine (aka Betadine) is the brown stuff they paint on your skin before surgery.
Cloud seeding with silver iodide
Silver iodide (AgI) is used for cloud seeding because it is SO insoluble in water that solid particles hang around in the cloud without dissolving (4). The stuff is crazy-insoluble. Look at the comparison of silver iodide and sodium iodide (NaI) in water at room temperature.
Sodium iodide - 1840 grams per liter
Silver iodide - 0.0000003 grams per liter
Looking at this in another way... it takes 1L of water to dissolve 1840 grams of sodium iodide. To dissolve the same amount of silver iodide it would take (if my math is correct - it never is) 6,133,000,000,000 (6.1 billion) liters (1.6 billion gallons) to dissolve that amount of AgI. That's 2,424 Olympic-sized swimming pools needed to dissolve 4 pounds of the stuff. So, it is not a coincidence that many iodine minerals are composed of silver iodide.
But insolubility itself isn't sufficient to seed clouds. Here is a nice explanation of how the process works at the atomic level.
QUIZ!
Can anyone explain why sodium iodide is so much more soluble in water than silver iodide? The prize is a pair of my sweaty socks. Do with them what you wish.
Iodine is sublime. And also sublimes.
Sublimation is an unusual phenomenon where a solid becomes a gas without first becoming a liquid. Some other examples of substances that sublimate include camphor, carbon dioxide, and naphthalene (mothballs). Here's how you can get iodine to sublimate:

Iodine is put in an Erlenmeyer flask. A cold finger (this can be a test tube containing ice) is put into the flask. When the iodine is gently heated, it sublimes (sublimates) and the gas forms extremely pure iodine crystals that collect on the cold test tube. Photo credit: Science Madness
Protection from nuclear radiation
In 2011 there was a worldwide shortage of potassium iodide following the Fukushima nuclear accident in Japan. While this was a terrifying event, it should be noted that the evacuation area around the plant was 12 miles. But this didn't discourage hysterical Americans on the west coast—more than 5,000 miles away—from panic buying potassium iodide, which protects your body against radiation poisoning. Within limits.
Potassium iodide (KI) is effective in protecting the thyroid gland against 131I – a radioactive isotope of iodine, which is one of the most abundant radioactive elements released in a nuclear accident. By looking at the chemical structure of the thyroxines it becomes evident why there was a mad dash for potassium iodide.
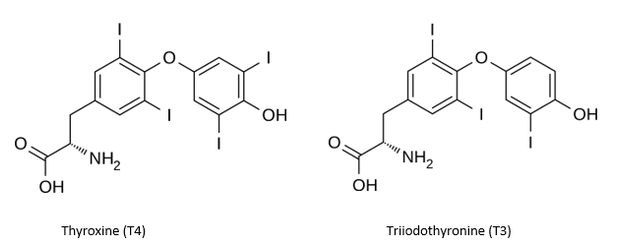
The chemical structure of two thyroxines. The number following the "T" is the number of iodine atoms in the molecule.
Thyroxines are thyroid hormones that contain a whole lot of iodine. Virtually all of the iodine in the body is found in the thyroid glands, which "import" dietary iodine/iodide and store it as thyroxines. So, when there is a nuclear accident131I heads straight for the thyroid gland (just like plain old stinky iodine) where it can easily cause thyroid cancer. This is the reason that large doses of potassium iodide are given to people who have been exposed to 131I. Potassium iodide swamps the thyroid gland and prevents the uptake of the 131I. But this only works as a preventative measure for 131I. Potassium iodide will not do anything to protect you from some of the other nasties that are released, for example, strontium-90 and cesium-137.
Blowing stuff up—not recommended, but still cool
Here are two simple reactions you don't want to try;
NH4OH (ammonia in water) + I2 -----------> NI3 (nitrogen triiodide)
Dry NI3 + anything ----------> Boom!
If one dissolves iodine in concentrated ammonium hydroxide, a dark purple solid will form, which can be collected by filtration. This is nitrogen triiodide and it is not to be toyed with. It's perfectly fine to handle the stuff as long as it remains wet. But when it dries, beware! Simply touching the dry powder, even a very small amount, with anything will produce a pants-soiling explosion. I know this because some maniac mined my entire lab with it when we were post-docs. For the story, which is hilarious, see Blowing Up Your Lab Mate Is A Bad Idea, But It Sure Is Fun.
I figure you've had enough of this by now, so I'll call it a day. Anyhow, it's time to (io)dine.
NOTES:
(1) My wife told me to work on my humility. I responded, "I'm so great that I don't need to." Slept on the couch.
(2) The difference between the two is a matter of semantics. The smallest unit of elements is a single atom. Since iodine exists as I2 it is technically correct to call it a molecule, not an atom. Don't lose any sleep over this.
(3) In case you care, here's a quote from Chemcool:
"Tennessine was made by a fusion reaction of element 20 with element 97: calcium-48 with berkelium-249. In the first synthesis of tennessine, calcium ions were formed into a beam in a cyclotron (a particle accelerator) and fired at a target layer of berkelium deposited 300 nm thick on titanium foil."
Piece of cake!