Nowhere is the silliness of our drug laws more evident than in the case of tetrahydrocannabinolic acid (THCA). The chemical, one of about a dozen found in hemp and marijuana, has no psychotropic activity, so it's legal, right?
Yes. Also no. And maybe. Hope that was helpful.
These are all correct, thanks to the tortuous, often conflicting, labyrinth of arbitrary laws that make up the mess that is called (but maybe not with a straight face) our "drug policy." There really isn't any semblance of policy regulating marijuana products. It's more like a dart board with the numbers missing, something I'll be writing about at a later date. Here's a tease.

Yeah, this really clears things up. Source: VIAA Hemp
THC
THC is short for delta-9-tetrahydrocannabinol, the primary intoxicant of cannabis. It remains classified as Schedule I by the geniuses at the DEA, the same category as heroin and illicit fentanyl. [Emphasis mine]
Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote.
Source: DEA Drug Scheduling
Seriously? Marijuana is in the same category as heroin? In what universe does this make sense?
Since state laws are all over the place I'm not going to try to make "sense" of them but feel free to do so yourself...
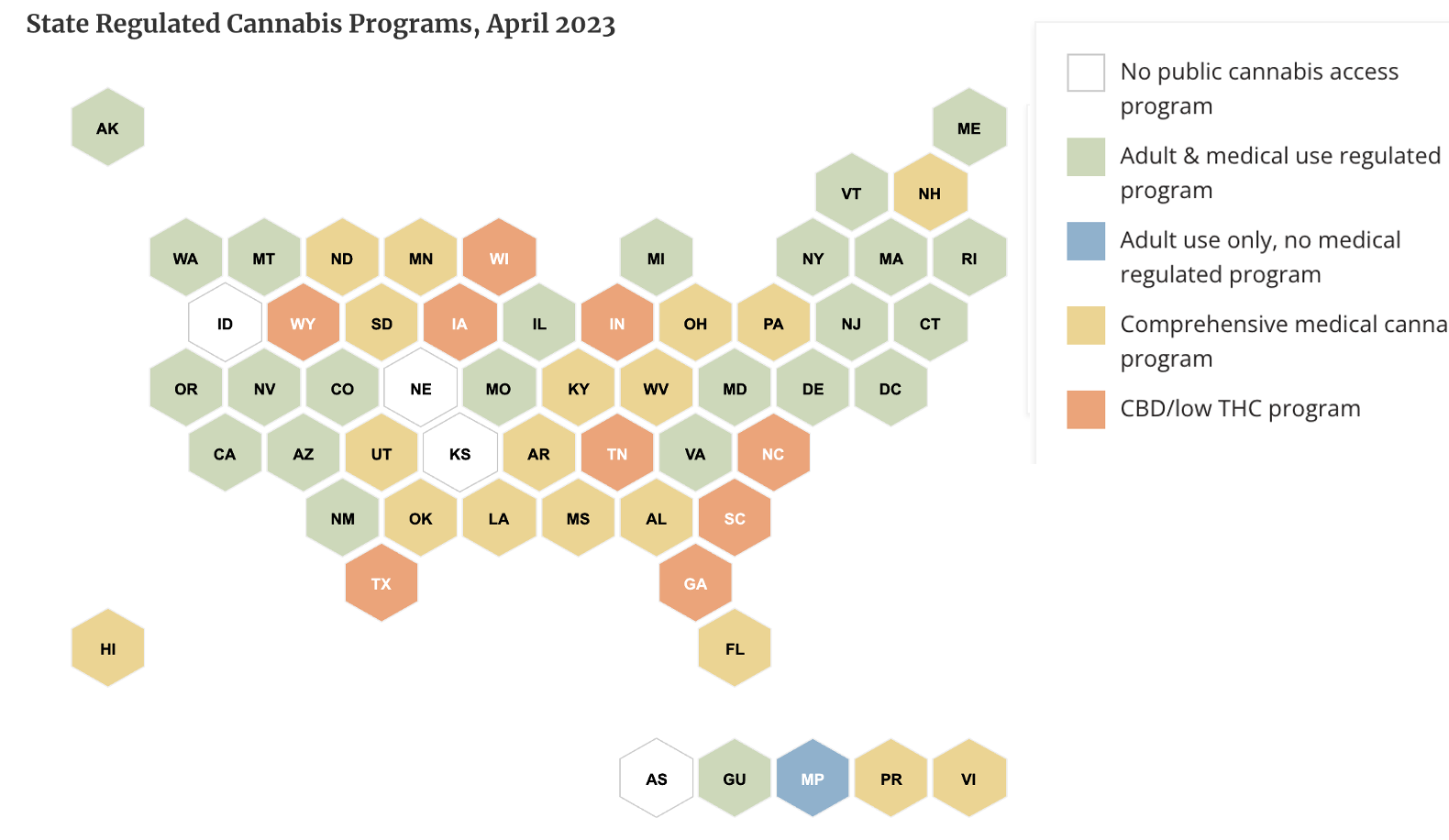
Source: National Conference of State Legislatures. As of April 2023
THCA (Tetrahydrocannabinolic acid)
Rather than getting bogged down in that mess, I'm going to discuss one cannabis component that is of particular interest: Tetrahydrocannabinolic acid. Why is it interesting? Three reasons:
- It has no psychotropic activity on its own.
- No one really seems to know if it is legal or not, but it could be technically illegal according to DEA regulations because of the Federal Analogue Act, which was passed in 1986, and as clear as mud:
"[A] controlled substance analogue is a substance which is intended for human consumption and is structurally or pharmacologically substantially similar to or is represented as being similar to a Schedule I or Schedule II substance.."
Source: DEA Drug Scheduling
- It has some interesting chemistry and we haven't had a Dreaded Chemistry Lesson From Hell® for way too long and Steve and Irving are getting antsy. And who among us doesn't want to know about the decarboxylation of beta-keto acids?
Be forewarned. It's time for...


Decarboxylation of ß-ketoacids (masochists only, please)
Usually, when you heat things, including most chemicals, they just get hot. But not in this case.
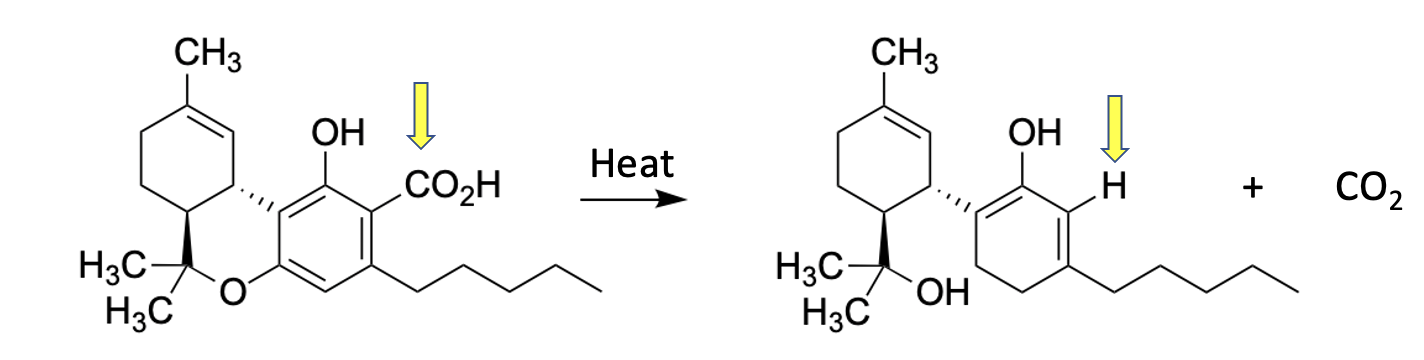
Decarboxylation of tetrahydrocanniboic acid forms 9-delta-THC
When THCA is heated, for example in hell, the carboxylic acid (yellow arrow), breaks down, losing CO2, and leaving in its place a hydrogen atom. This is a well-known reaction in organic chemistry, which is called the decarboxylation of a ß-ketoacid – a fact that will be thoroughly useless in your life, no matter how long you live. Here's the standard example:
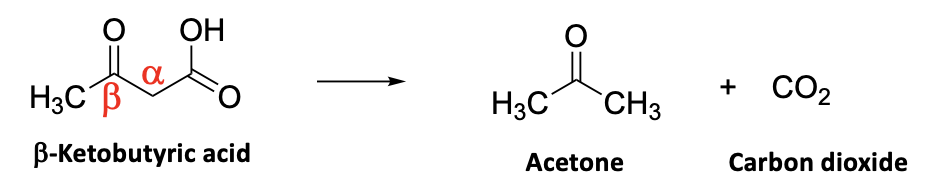
OK, that's just peachy, but if you look at the structure of THCA there isn't any ß-keto acid, right? So how can heating the damn stuff make it turn into THC??
Just another reason why people hate organic chemistry
Organic chemistry is simply a set of rules. Once you learn them you've got it down, right? No. Not right. This is because there are rules about rules, some of which are obvious. Many are not. Here's one that isn't.
But before you look, please remove the following items from your home so you won't be tempted to use them.

Don't say I didn't warn you. For crazy bastards anyone who made it this far...
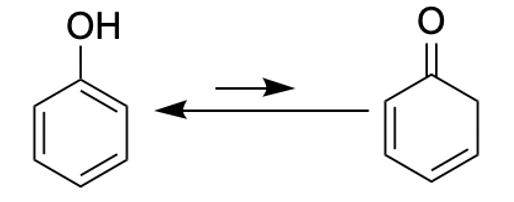
Phenol (left) can exist in two forms interchangeable forms. The enol form (left) predominates, but there is also a teensy bit of keto form (left) in there too, which just happens to be a ß-keto acid. This explains why THCA can undergo decarboxylation when heated.
\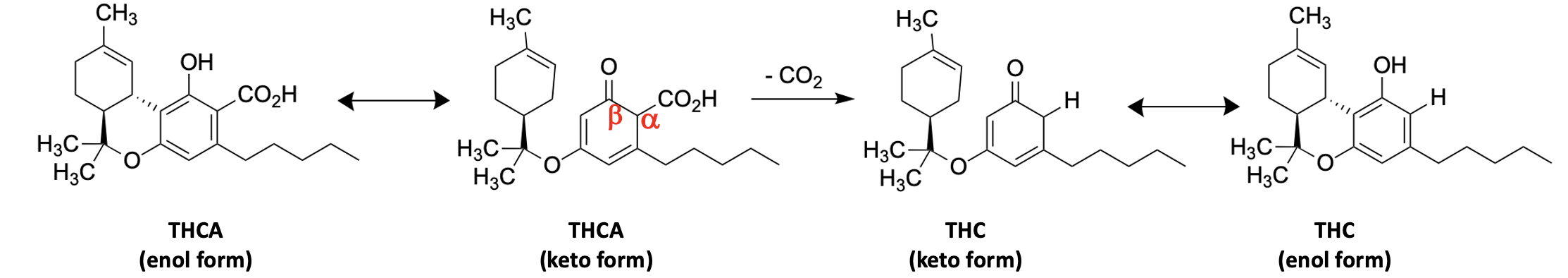
THCA in its enol and keto forms. Note that the keto form is a poorly-disguised beta-keto acid, which means that it loses carbon dioxide upon heating. It does, giving the keto form of THC, which immediately rearranges to THC.
This mercifully ends The Dreaded Chemistry Lesson From Hell®. I doubt many of you are mourning this development, but don't blame me. You asked. (1)
It might be time to break out the THCA. Just remember to heat it.
NOTE:
1) I'm not kidding. I do get requests from more than a few people to do these wretched articles. No accounting for taste. After all, some people love kale.




