Misguided COVID-minimizers like to say that COVID-19 is no worse than a cold that lasts a few days and then disappears without any sequelae. They’re so wrong, and the evidence of that continues to mount.
Alzheimer's disease
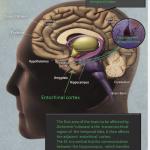
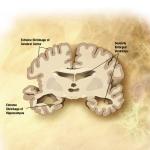
One of the significant hurdles in the new FDA therapies for Alzheimer’s Disease (AD) is identifying those patients in the early stages of the disease when alterations in the brain have begun, but early dementia may be problematic to diagnose
Alzheimer's drugs, at least the experimental ones, are a dime a dozen. Maybe less.
The Food and Drug Administration on July 6 granted full approval to the first therapy for Alzheimer’s that slows the cognitive decline associated with the disease.
Here are the eleven from Nature, plus two bonus picks from me.
I spent 15 years as the FDA’s “biotechnology czar” at a time when many of the biopharmaceutical companies were small startups needing guidance as they negotiated the regulatory maze.
Statin use is ubiquitous, and a careful read of the literature will demonstrate some small percentage of each clinical trial having increased or decreased risk of dementia and Alzheimer's Disease (AD).
Biogen announced that its current candidate for an Alzheimer’s drug was ineffective, resulting in an 18 billion dollar loss in valuation – that number should provide a sense of both the need and market for an effective treatment.