biotech
Interest rates are a significant factor in the health of any economy.
This week is full of news relevant to those of us concerned about the future of antibiotics. The pharmaceutical industry is rebelling against the US plan to have Medicare negotiate drug prices.
A growing chorus of political commentators and social scientists claims that the West is using genetically engineered crops to “re-colonize” developing countries.
This article was originally published at Leaps Mag. It is reprinted with permission.
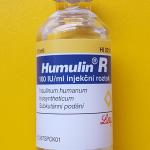
October 31st will mark the 37th anniversary of one of biotechnology’s most significant milestones -- the approval by the FDA of human insulin synthesized in genetically engineered bacteria. It launched a revolutionary ne
The protection of intellectual property is vital to innovation. If anyone can just take something you created -- be it a song or a drug -- without proper compensation, there would be little reason to develop new things.
The protection of intellectual property is one of the biggest challenges facing the technology industry.
In a piece published today in the journal Science Translational Medicine, researchers present a proof-of-concept study maintaining they designed “an accessible solution that uses speakers and microphones within existing smartphones to det