OK, this is probably not as noteworthy as, say, National Dog Fart Awareness Day (No - I'm not kidding; it's April 8th). But worry not. For all you dorks learned individuals who, for some strange reason, enjoy The Dreaded Chemistry Lesson From Hell®, this article will satisfy your salacious urges while also having plenty for unemployed losers history majors.
Yessiree, at ACSH, we do it all – we're your one-stop emporium for knowledge, useful or otherwise!
Now that we are done with that vulgar bit of self-promotional tripe, let's discuss ether. It's really quite interesting!
A Little History
Ether, short for diethyl ether, is one of the most important chemicals in history and its use as a general anesthetic is arguably one of the greatest discoveries of all time (1). Surgery before anesthesia was a living nightmare. Here is one especially gruesome example of a woman named Fanny Burney, undergoing a mastectomy in 1811:
When the dreadful steel was plunged into the breast … I needed no injunctions not to restrain my cries. I began a scream that lasted unintermittently during the whole time of the incision … so excruciating was the agony … I then felt the Knife [rack]ling against the breast bone – scraping it.
Source: David Liley in The Conversation
Far too late for Ms. Burney, on October 16th, 1846, William T.G. Morton, a Boston dentist, demonstrated the first use of what was then called sulfuric ether (see chemistry section) during an operation at Massachusetts General Hospital. Morton, arguably the world's first anesthesiologist, administered his invention (2) while Dr. John Warren, a famous surgeon, removed a tumor from a patient's neck.

William T.G. Morton DDS. Source: Wikipedia
Exactly 60 years later, on October 16th, 1906, Ether Day was born in an article in the Journal of the American Medical Association.

Source: JAMA. 1906; XLVII(15):1199, Anon
You Know What's Next

Even though it is one of the most volatile solvents (boiling point 95oF), it is highly flammable, and if you leave it around too long will be only too happy to blow up; ether is a staple of synthetic organic chemistry – a very commonly-used solvent. Every organic chemist has used it at one time or another. And we can detect its distinctive sweet mostly-pleasant scent in our sleep.
A little nomenclature... Technically, ether is not a chemical; it is a class of organic chemicals that contains a Carbon-Oxygen-Carbon (C-O-C) fragment. But the term "ether" is also a universal abbreviation for diethyl ether. What's going on? In this case, (only) diethyl is assumed when "ether" is used. But it's not that simple. Both carbon atoms must be bound to either hydrogen or an alkyl group or a substituted alkyl group (Figure 1).
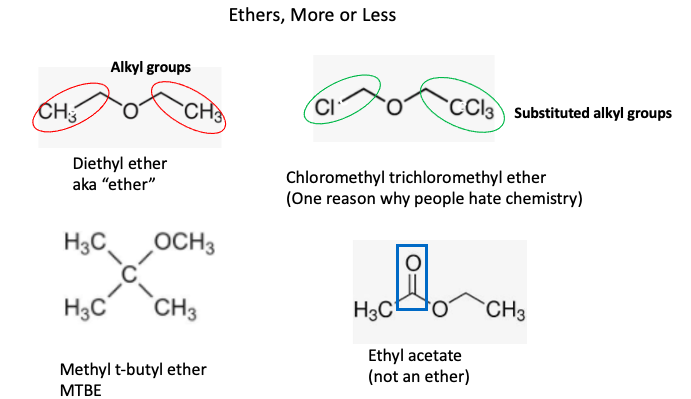
Figure 1. Diethyl ether contains two alkyl groups, in this case, both are ethyl groups. You can replace hydrogen atoms on the alkyl groups and the chemical is still part of the ether class. Hence, the ghastly-looking name chloromethyl trichloromethyl ether. You may recall the term MTBE from the gas station. It was an anti-knock component of gasoline until it was banned in 2002 because it is water-soluble , found its way into groundwater, and stayed there. Although ethyl acetate looks a bit like ether, it isn't because that stupid thing in the blue box is a carbonyl group, not an alkyl group.
Why "Sulfuric Ether?"
There is no such thing as sulfuric ether. The name comes from how it was made – by heating wine with sulfuric acid. (Figure 2). While a number of acids will work, this exact reaction, first performed in 1540, is today's industrial process for producing ether, although alcohol, not wine, is used. How crazy is this?
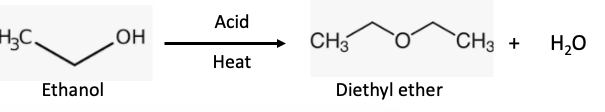
The synthesis of ether from ethyl alcohol is a dehydration reaction (water is formed)
Ether Goes Boom
If you're using ether in the lab, you better damn well put down the date you opened it, or the safety idiots inspectors are going to blow a fuse. Why? Because like bread, ether goes "stale." Only in this case, "stale" has a rather different meaning. You really don't want ether sitting around with oxygen in the can (Figure 2).
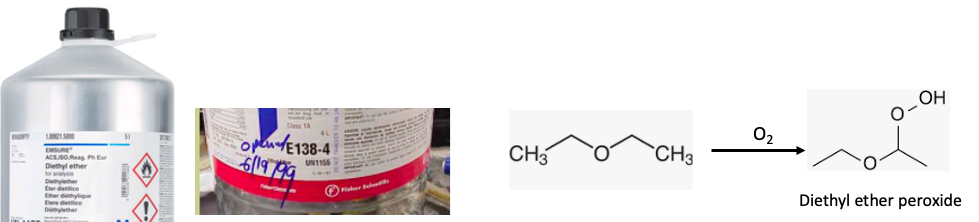
Photo credit: Sigma-Aldrich
Figure 2. (Left) A new can of ether and one that has been opened. Note the date written on the can. An opened can of ether must be disposed of after six months. (Right) The reaction of ether with oxygen to form diethyl ether peroxide. I do not recommend trying this as a hobby.
Or this can happen...

An ether peroxide explosion in California. Photo credit: Sean Scully/Weekly Calistogan, Napa Valley Register
Why do peroxides blow up? "Why Stuff Explodes" (2016) is an excellent place to start.
Get High With a Little Help From My Chemist Friends
Like this article, ether will put you to sleep. And like every other drug or chemical that can alter your mental state, it has been abused.
Ether was especially popular in Ireland in the 1800s, where it was considered a more socially acceptable alternative to alcohol when anti-alcohol campaigners were getting going. (Sound familiar?) People actually drank the stuff. Perhaps my aversion to this comes from my first career as an organic chemist, where we rarely grabbed a random bottle of some chemical off the shelf and took a swig. (More commonly, because of its extreme volatility, ether is inhaled.) Ireland declared it a poison in 1890.
The literature contains scattered examples of ether addiction (probably not real), accidental poisonings, an apparent suicide by asphyxiation, and overdose deaths, which weren't uncommon since ether was used as a general anesthetic until about 1960. The DEA classifies it as a "listed chemical" – not because it can be abused, but because it is commonly used in the synthesis of street drugs, such as methamphetamine and cocaine.
Good Night
I figure that most of you are asleep by now (or in a coma). If not, you could (but should not) try some ether. There is even a cool delivery device that was patented in 1897: Woodling's Ether Cone. Probably not on eBay.

Photo credit: JAMA
Gauze lined; ready for use; clean, convenient. Use once and throw away. It consists of a strong paper cone and separate gauze lining, with impervious tissue paper between. The cone is folded in, around its mouth, so as to more securely hold the lining and also obtain a smooth surface to come in contact with the patient's face. The trough formed by this fold catches any surplus ether and it is reabsorbed by the lining instead of running out over the patient's face. These cones are cheaper than cleaning and replacing the lining in any permanent inhaler, besides saving the cost of the latter. A clean, new cone for each patient.
Dr. M.E. Woodling in the Journal of the American Medical Association, December 4, 1897
Sleep tight!
NOTES
(1) Chloroform was also used at that time as a general anesthetic, mostly in Europe. I cannot adequately express what a bad idea this is.
(2) There is some debate about who the inventor is. An article in BMC Anesthesiology claims that the actual inventor was Crawford Williamson Long, a physician. The origin of the conflict is that Long used ether four years earlier but didn't publish it until 1849. And an article in Scientific American doesn't even mention either of them. I'll let them fight it out.




