Summer is around the corner, so in addition to the usual annoyances, mosquitos, ticks, visiting relatives (worse because insect sprays don't work very well), there is always the delightful possibility of brushing up against the always-hideous poison ivy. Poison ivy is some nasty -ass stuff. But if you read this nasty-ass article to the end at least you will no longer need to scratch your head about why you're scratching the rest of you. It's all about chemistry and toxicity.
Experienced medicinal chemists (1) will usually develop an ability called "eyeball toxicity" - the ability to look at the chemical structure of a molecule and make a pretty good guess about whether the molecule will be toxic. But this only works in one direction. It is not difficult to look at a list of chemicals and predict which ones will be toxic (2), but it is not easy to look at the same list and predict the chemicals that will not be. The reason that the first task is relatively simple is because of toxicophores - fragments of a molecule that are known to produce toxicity when they are found within a wide variety of chemical structures. Here's an example (Figure 1).
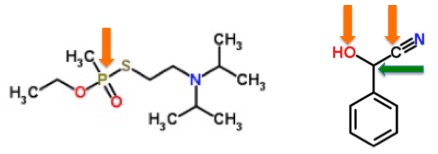
Figure 1. The toxicophore of the deadly neurotoxin VX (Left) is the phosphorous atom (orange arrow) bound to one sulfur and two oxygen atoms. The phosphorous is known to react with acetylcholinesterase - the enzyme that is responsible for maintaining the right level of acetylcholine - a critical neurotransmitter in the brain. The toxicophore in benzaldehyde cyanohydrin (Right) consists of a carbon atom (green arrow) which has both a hydroxyl (OH) group and a cyanide (CN) group (orange arrows) bound to it. Cyanohydrins break down to form cyanide, chemically and enzymatically. This is the reason that the quack cancer drug laetrile poisons people. (See Laetrile, Amygdalin, Vitamin B17: Different Names, Same Poison.) An experienced medicinal chemist should easily be able to determine in advance that both of these molecules are going to be toxic.
Now, let's take a look at three naturally occurring molecules, all with certain characteristics in common (Figure 2).
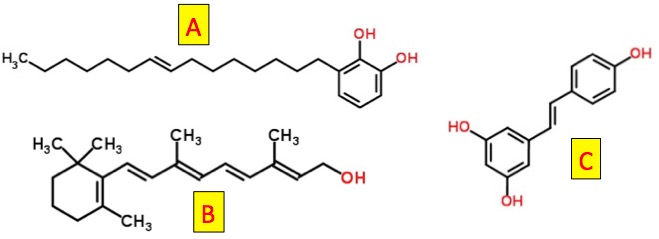
Figure 2. Which of these is toxic? Molecule A contains a long chain of carbon atoms, one carbon-carbon double bond, and two hydroxyl groups. Molecule B also a long chain of carbon atoms, five carbon-carbon double bonds, and one hydroxyl group. Molecule C contains one carbon-carbon double bond and three hydroxyl groups. Which is toxic?
The answer is "who knows?" All three molecules have common structural elements. But there is no obvious toxicophore. So, unless you happen to recognize the structures there is no way to tell which of these will be good, bad or neither.
The answer - one good, one bad and one neither. Molecule A bad. It is called urushiol (3). It is the chemical in poison ivy that is responsible for doing all kinds of hideous things to you. Molecule B is good. It is vitamin A, without which you will go blind. Molecule C is useless. It is called resveratrol. Although it was touted as an antioxidant therapy to fight heart disease, inflammation, and aging, it did none of these, something that cost GlaxoSmithKilne $720 million to find out.
So, how does a benign-looking molecule like urushiol induce the torture that poison ivy sufferers must endure? It turns out that urushiol has some properties that make it quite efficient at this, and these properties arise from its chemical structure. You just need to look more carefully and it starts to make sense (Figure 3).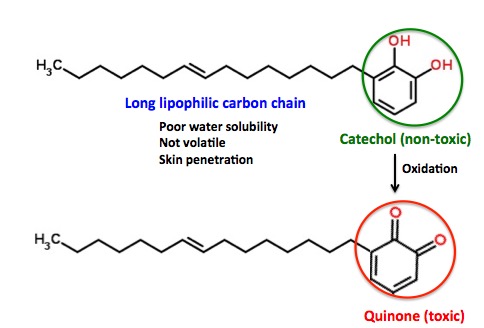
Figure 3. Structural elements that determine the toxicity of urushiol. (Top) Urushiol can be dissected into two fragments: 1) A 15-carbon lipophilic (fat-loving, oily) chain, which makes the molecule insoluble in water. This is why ordinary soap will not remove it from the skin, and also why it sticks to gardening tools, dogs fur... This same property enhances absorption into the skin and makes the molecule large enough that it will not evaporate. 2) A catechol (1,2-dihydroxybenzene) ring. This is the business end of the molecule, but not as is. Catechols are easily oxidized to ortho-quinones, which are chemically reactive and toxic. The quinone then reacts with proteins in the membrane of the skin resulting in the modification of the skin proteins. This modification triggers a complicated series of immune system events, which results in an inflammatory response - the rash.
Guess what? It's time for a quiz!!!!

Photo: Bureau of Betterment
There is a fruit that some people are allergic to because it is a close relative to poison ivy. What is it? (4)

But be forewarned. Wiki cheaters will not be tolerated on this site. Photo: Trip Advisor
So, I wish you an itch-free summer. But if you're not careful (three leaves and shiny) a toxic natural chemical, one of thousands of such chemicals out there will make your life miserable. Maybe even as bad as watching an episode to the Dr. Oz show.
Nah.
NOTES:
(1) Medicinal chemists are trained as synthetic organic chemists, which means that we are capable of transforming one molecule into another in the most efficient way by choosing the right reaction(s). Once organic chemists start pharmaceutical research the synthesis becomes merely a tool to make molecules with specific properties and activities. Medicinal chemistry is the essence of drug discovery.
(2) No one will get it right all the time. Different chemists have used or read about a large variety of different chemicals in their careers. So, Chemist A may recognize a toxicophore that Chemist B is not familiar with.
(3) There are a number of closely related urushiols, differing only by the number and position of the double bonds in the lipophilic chain. They are all very similar in properties.
(4) Mango




