
In 2016 I wrote about a group at the Kyoto Institute of Technology in Japan that identified a new bacterium that was capable of "eating" polyethylene terephthalate, aka PET— the polyester plastic that is used for bottled water and other drinks. The Kyoto scientists named the bug Ideonella sakaiensis.
Perhaps most interesting was that Ideonella sakaiensis wasn't some ancient species that just happened to have some freakish properties that, by some amazing coincidence, was capable of breaking down a plastic first discovered in the 1940s. No – Ideonella was isolated from water near a recycling plant. Humans had inadvertently created it by supplying a bazillion water bottles. Bacteria in those waters evolved so that they could metabolize PET and use it for a food source, giving them an adaptive advantage over other bugs in the area.
How can bacteria "eat" plastics? They do so using an enzyme called PETase. In order to understand how this works, (more than) a little organic chemistry is needed (my apologies). This is because, although organic chemistry and biochemistry work under very different sets of conditions, the reactions are the same.
Figure 1 shows the basic structure of an ester – the reaction product of a carboxylic acid, in this case, benzoic acid, and alcohol, ethanol (which, if you're a non-scientist, you'll be desperately over-consuming by the time you finish this wretched article). The ester bond that is formed is shown with a green arrow. Water is a byproduct of the reaction.
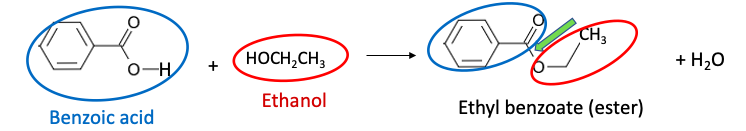
Figure 1. A carboxylic acid reacts with an alcohol to form an ester
The chemistry is the same when the molecule with the carboxylic acid contains a second one, as is the case with terephthalic acid, and the alcohol has two hydroxyl groups (ethylene glycol) but the product is very different (Figure 2). In this case, when ester bond (green arrow) #1 forms between ethylene glycol and terephthalic acid there is still another carboxylic acid in the molecule that can form another ester bond (#2) with another molecule of ethylene glycol. This new molecule now contains and extra alcohol which reacts with another molecule of terephthalic acid (#3), and so on. This process continues to form long chains of esters – polyesters.
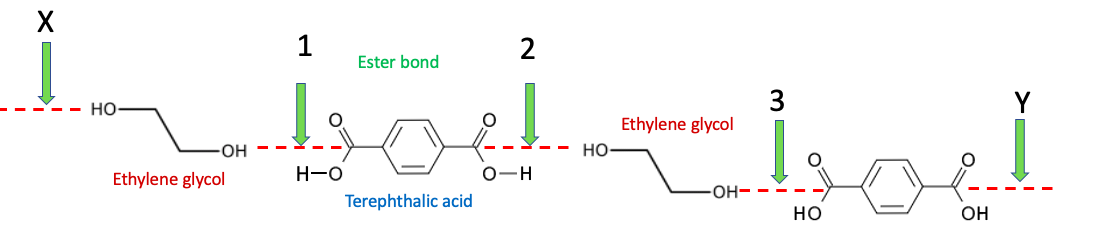
Figure 2. "Double" alcohols (ethylene glycol) react with "double carboxylic acids" (terephthalic acid) to form long chains (polymers) called polyesters.
Another way this is portrayed is in Figure 3. Repeating units of terephthalic acid (blue oval) and ethylene glycol (red oval) are joined by ester bonds (green arrow). The letter "n" outside the bracket indicates the number of repeating units, usually large.
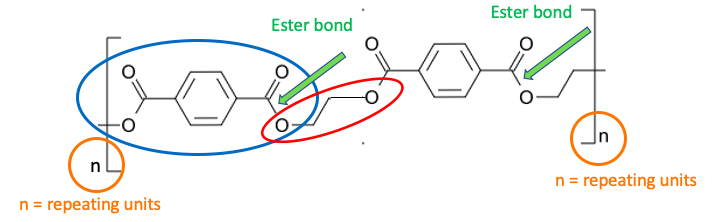
Figure 3. The structure of a polyester.
What does this chemistry have to do with the degradation of water bottles?
Esterification reactions are reversible (a process called hydrolysis– the opposite of esterication). These can be done chemically or enzymatically. Enzymes are much faster. This where the bacteria come into play. They produce enzymes called esterases (in this case the specific esterase is called PETase), which break the ester bonds, reverting back to the alcohols and carboxylic acids from which they were made. I'm not drawing any more of these damn structures, so just go back to Figure 2 and run it backwards. (Any complaints? Take it up with our quality control team - me.)
So, could Ideonella be used to devour the countless plastic bottles that are polluting the earth? Not really. According to the Kyoto scientists it took "6 weeks at 30°C [86º F] to fully degrade a thumb-nail-sized piece of PET.” While this may be faster than a crosstown bus in Manhattan, it's not even remotely close to being useful.
In 2018 Professor John McGeehan, the director of the Centre for Enzyme Innovation at Portsmouth University, working with an international group of scientists discovered a similar version form of PETase enzyme called MHETase (short for monohydroxyethyl terephthalate hydrolase). MHETase also degrades PET faster. When both enzymes were used together...
“What actually turned out was we improved the enzyme, which was a bit of a shock... It’s great and a real finding.”
Prof John McGeehan, at the University of Portsmouth, UK
MHETase, performs a similar function – hydrolysis of an ester bond – but operates on a much smaller fragment of PET:
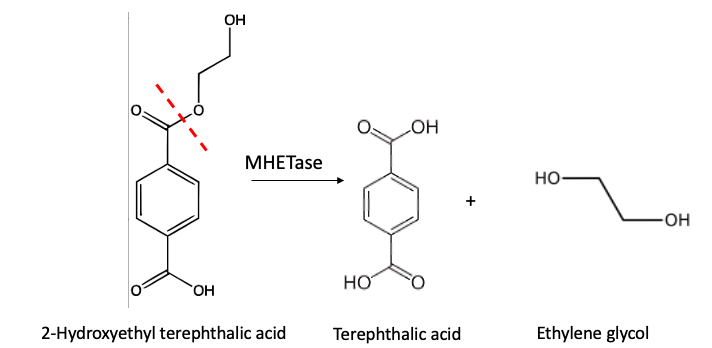
Figure 4. Hydrolysis of 2-hydroxyethyl terephthalic acid (aka mono-terephthalic acid) by MHETase. Same chemistry but on a much smaller molecule.
Even more interesting is what happens when the two enzymes are physically connected to one another via a chemical bond. This is described in a new PNAS paper by McGeehan and colleagues. When the two enzymes are mixed together. PETase chomps up the large polymer and the MHETase completes the hydrolysis by performing the last step. But when the McGeehan group linked the two enzymes together to form a dimer it worked a six-times faster than PETase alone.
The enzymology behind this discovery is beyond the scope of this article, but there is a nice summary of the work on the Science Times site.
While this new method alone isn't likely to put a dent in plastic pollution (reaction speed and scale) it is an important (and cool) concept – linking two enzymes to make each of them more effective – that it will no doubt be applied to other biodegradation research, as this as scientists continue to search for innovative ways to clean up some of the mess we've made.



