The Frank R. Lautenberg Chemical Safety for the 21st Century Act amends the Toxic Substances Control Act (TSCA) and was signed into law June 22, 2016. It created a mandatory requirement for EPA to evaluate existing chemicals with clear and enforceable deadlines, to do so in a transparent fashion, and to do so using risk-based chemical assessments rather than rely on simple epidemiological correlations.
EPA selected the first 10 chemicals to undergo risk evaluation under the amended TSCA and to make those understandable for the public, the American Council on Science and Health is producing risk-based evaluations of each, which will then be compiled into a free downloadable book for consumers.
What is Dichloromethane (DCM)?
Methylene chloride, also known as dichloromethane (DCM), is a colorless liquid with a mild, sweet odor which evaporates easily but does not readily burn. It is widely used as an industrial solvent and as a paint stripper and can be found in certain aerosol and pesticide products and may also be found in some spray paints, automotive cleaners, and other household products. Methylene chloride is also used in the manufacture of photographic film. It is made from methane gas or wood alcohol, it does not occur naturally in the environment.
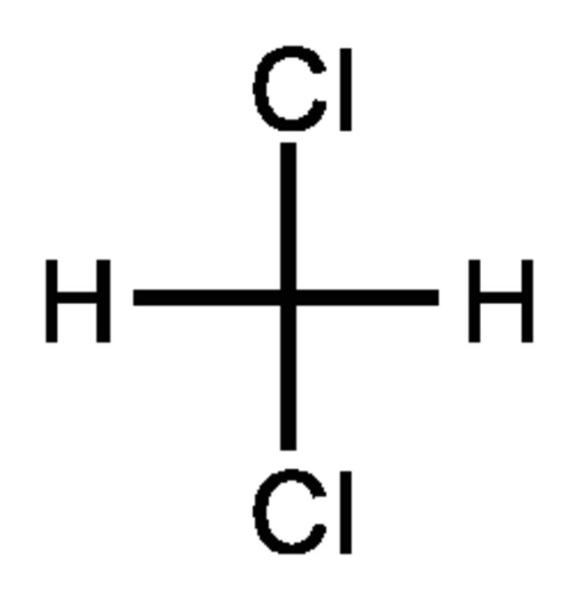
The chemical structure of methylene chloride is very simple as shown at the US National Library of Medicine’s Toxnet database (NLM, 2018a). It is one of the simplest chlorinated hydrocarbons, made by substituting two chlorine atoms for two hydrogen atoms on the methane molecule (formula CH2Cl2). This simple structure and ability to be metabolized lead to its principle effect in experimental animals, that of liver and kidney toxicity at high levels of exposure (ATSDR, 2000). EPA (2011) indicates that the most common effect in humans is on neurological function as seen in numerous case reports and experimental studies.
Exposure to DCM
According to EPA (2011), methylene chloride in the environment will partition mainly to air and in the form of a vapor. Although some of the methylene chloride released to soil or water is expected to volatilize to air, it is expected to be highly mobile in soil and may migrate to groundwater. In soil or water, methylene chloride may biodegrade either with or without air.
According to ATSDR (2000), inhalation of methylene chloride from ambient air is the predominant exposure route for the general population in the United States. Based on a 1979 monitoring data and a daily air intake of 23 m3 by an adult, the average daily doses of methylene chloride from ambient air in three U.S. cities ranged from about 30 to 300 µg/day. ATSDR (2000) also reported methylene chloride detection in surface water, groundwater, and finished drinking water throughout the United States, ranging from 0 to 3,600 ppb in the 1980’s, while mean concentrations of generally less than 1000 ppb were reported in numerous drinking water supplies in the 1970’s. In contrast, ATSDR (2000) found little or no information on levels of methylene chloride in food or soil and no reliable monitoring data for the levels of methylene chloride in contaminated media at hazardous waste sites.
Individuals using consumer products containing substantial amounts of methylene chloride have the potential for high exposure to this compound. ATSDR (2000) also states that people living near industrial or hazardous waste sites with higher than average levels of methylene chloride in the air or water would have a potential above-average exposure. In addition, workers in the industries involved with methylene chloride manufacturing, paint manufacturing, metal cleaning, polyurethane foam manufacturing, plastics and adhesives manufacturing, ink use, pharmaceuticals, construction, and shipyards have the highest potential exposure to methylene chloride.
Dichloromethane Health Effects
According to both ATSDR (2000, 2010) and EPA (2011) methylene chloride is rapidly absorbed following inhalation, oral, and dermal exposures in both humans and experimental animals. Absorbed methylene chloride is metabolized via two metabolic pathways. One metabolism pathway produces carbon monoxide (CO) and carbon dioxide (CO2), while a second metabolism pathway produces carbon dioxide by way of formaldehyde. Both pathways can give rise to toxic forms of methylene chloride, with the formation of formaldehyde being linked to increased cancer. The CO formed can also bind to hemoglobin to form carboxyhemoglobin (COHb), resulting in a reduction in the oxygen carrying capacity of the blood. Methylene chloride is eliminated in humans and animals mainly through exhalation either of the parent compound or as the two primary metabolites (CO2 and CO). Elimination via the urine is reported to be generally small in exposed humans, but urine and feces are also elimination pathways in experimental animals.
Like exposure to any chemical, toxicity of methylene chloride depends on the level to which one is exposed and the length of time of exposure. Both ATSDR (2000, 2010) and EPA (2011) have an abundance of health effects information on methylene chloride. Neurological effects, including central nervous system (CNS) depression and resulting narcosis, respiratory failure, and heart failure, are common features in numerous case reports and controlled human studies involving single, high level exposures to methylene chloride. Unfortunately, relatively less is known about the potential long-term neurological effects of chronic exposures to humans, which raises concerns following chronic exposure. In contrast, longer term but limited data suggest little evidence of cardiac damage related to lower levels of methylene chloride exposure in the cohort studies of exposed workers. Also, limited data available in workers, exposed to estimated average exposures of 400 to 500 ppm methylene chloride for more than 10 years, provided no clear evidence of liver damage. Available epidemiological data suggest an association between methylene chloride and brain cancer, liver cancer, and specific hematopoietic cancers, but not lung cancer. Thus, methylene chloride, at least at high concentrations, may cause cancer in humans.
Both ATSDR (2000) and EPA (2011) note that acute toxicity, such as central nervous system depression, irritation, liver toxicity, neurotoxicity and death occur in experimental animals given high concentrations over short time by both inhalation and oral routes of exposure. Experimental animals exposed by the oral route for short-term and longer-term exposures have shown the liver and the nervous system as the most sensitive targets for noncancer toxicity. Longer-term inhalation exposures in experimental animals have also identified the CNS, liver, and lungs as potential non-cancer toxicity targets. Oral exposures to methylene chloride in experimental animals also caused liver tumors while inhalation exposures resulted in benign mammary gland, liver, and lung tumors.
DCM Safe Levels
Federal and state regulations and recommendations are often expressed as a safe or virtually safe level, that is, a level of a substance in air, water, soil, or food that is not expected to cause any adverse health effect, even in people who are sensitive to the chemical’s effects. These safe levels are usually based on information from experiments with animals (usually rodents) at much higher levels of the chemicals than humans would typically encounter, so they are very conservative.
Such higher animal exposures are used to see what the adverse health effects might be in humans at lower levels of exposure. Scientists then estimate the safe or virtually safe level with safety/uncertainty factors or other methods of protective extrapolation. Sometimes safe levels differ among federal and state organizations because of different choices in assumptions for human exposure, animal study, or underlying method. Other times these recommendations differ because new science develops that suggests different levels are toxic or safe. ATSDR (2000) and NLM (2018b) give examples of these differing recommendations for methylene chloride among government organizations. Recommendations and regulations are also updated periodically as more information becomes available.
Why Is EPA Looking At Methylene Chloride Under The Lautenberg Chemical Safety Act?
EPA scientists looked at the likely routes of exposure to methylene chloride in the environment to develop exposure scenarios, or pathways, of how the public comes into contact with it. The amount of methylene chloride in these pathways will then be compared to its safe or virtually safe dose. If human exposure in the pathway is at or below this safe or virtually safe level, then the amount of methylene chloride exposure from this pathway is not considered to be a human health risk.
If exposure is above this safe or virtually safe level, then the pathway might be considered as a possible health risk; (1) several pathways may be added together to suggest a health concern. In either event, regulations might be developed to lessen the exposure of methylene chloride from this pathway(s), or the use of methylene chloride in this pathway might be banned. See also EPA (2018) for specific questions related to the assessment of methylene chloride under the new LCSA.
Controversy Over Dichloromethane
EPA (2011) concluded that methylene chloride shows “some evidence of carcinogenicity” in male rats (benign mammary gland tumors) and “clear evidence of carcinogenicity” in female rats (benign mammary gland tumors) and in male mice (liver tumors) and female mice (liver and lung tumors). More specifically, EPA (2011) regards methylene chloride as a possible cancer-causing substance, and that it forms cancer by mutation of DNA.
However, outside scientists question EPA’s choice of the mouse as the most appropriate animal species for the assessment of risk to humans.
The synopsis of tumor formation is based on chromosomal aberrations, formation of micronuclei (i.e, small pieces of DNA outside the normal nucleus), and DNA damage that correlate with metabolism of methylene chloride to more toxic forms. EPA goes on to conduct its extrapolation to human risk in a conservative linear approach (where any concentration of methylene chloride has the potential to cause a cancer). Other studies on methylene chloride, particularly in mice, have raised concern over its potential to cause cancer in humans, and some of the studies in rats and hamsters found no exposure-related cancer responses. The difference in susceptibility to cancer formation among mice, rats and hamsters likely reflects differences in the formation of toxic metabolites among these species.
Specifically, whereas EPA (2011) considers mouse liver tumors to be relevant to humans and that a mutation mode of tumor formation is appropriate in the absence of alternatives, others note that methylene chloride is not a human carcinogen, since the different metabolic pathway of mice makes it uniquely susceptible (Starr et al., 2006). Thus, lung and liver cancer seen in mice does not apply to humans, as demonstrated by the inconclusive human epidemiological studies., including occupational studies at high concentrations. Starr et al. (2006) go on to note that the observed mouse tumor responses are also not directly proportional to the concentration of methylene chloride in air, but rather reflect the formation of internal metabolites. Thus, EPA’s use of a linear extrapolation, even assuming that the mouse is its reasonable surrogate for humans, is also not appropriate. EPA’s response to these concerns is that its derivation of a cancer risk estimate addresses high-risk subgroups of humans (Schlosser et al., 2015). To date, this controversy has not been resolved.
Carcinogenicity claims aside, the American Council on Science and Health has recommended it be banned for home paint stripping use. There is no way to significantly reduce exposure to methylene chloride if it is being used to strip paint, especially in a non-industrial setting, such as homes. It takes a lot to do the job completely, and it is so volatile that it is difficult not to inhale some unless you are using a commercial full-face respirator, something that do-it-your-selfers are unlikely to do.
"Methylene chloride is arguably the most dangerous of all the solvents sold at Home Depot," said Dr. Josh Bloom, Senior Director of Chemical and Pharmaceutical Sciences at the American Council on Science and Health. "Chemists use it all of the time, but we do so in fume hoods. Some argue it's not necessary to ban it, but this is not a knee-jerk chemophobic response by EPA. There is real risk here."
More analyses in our series on the Lautenberg Chemical Safety Act compounds:
ACSH Explains: What's The Story On Dioxane?
ACSH Explains: What's The Story On Trichloroethylene (TCE)?
NOTE:
(1) Small excesses of the safe or virtually safe dose are seldom cause for concern since the safety levels are developed from conservative assumptions, including the use of safety factors that tend to exaggerate risk and exposure pathways that tend to exaggerate exposure.
REFERENCES:
Schlosser PM, Bale AS, Gibbons CF, Wilkins A, Cooper GS. 2015. Human health effects of dichloromethane: key findings and scientific issues. Environ Health Perspect 123:114–119; http://dx.doi.org/10.1289/ehp.1308030.
Starr TB, Matanoski G, Anders MW, Andersen ME. 2006. Workshop Overview: Reassessment of the Cancer Risk of Dichloromethane in Humans. Toxicol. Sci., 91 (1): 20–28. Available at: https://doi.org/10.1093/toxsci/kfj145
U.S. Agency for Toxic Substances and Disease Registry (ATSDR). 2000. Toxicological Profile for Methylene Chloride. September. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=234&tid=42.
U.S. Agency for Toxic Substances and Disease Registry (ATSDR). 2010. Addendum to the Toxicological Profile for Methylene Chloride. July. Available at: https://www.atsdr.cdc.gov/toxprofiles/methylene_chloride_addendum.pdf
U.S. Environmental Protection Agency. 2011. Toxicological Review of Dichloromethane (Methylene Chloride). Integrated Risk Information System (IRIS). National Center for Environmental Assessment. EPA/635/R-10/003F. Washington D.C. available at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0070....
U.S. Environmental Protection Agency. 2018. Problem Formulation of the Risk Evaluation for Methylene Chloride (Dichloromethane, DCM). Office of Chemical Safety and Pollution Prevention. EPA Document# EPA 740-R1-7016. Available at: https://www.epa.gov/sites/production/files/2018-06/documents/mecl_proble....
U.S. National Library of Medicine. 2018a. ChemIDplus: Methylene Chloride Toxnet database. Found at: https://chem.nlm.nih.gov/chemidplus/rn/75-09-2.
U.S. National Library of Medicine. 2018b. International Toxicity Estimates for Risk (ITER) database. Found at: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~kZPiEp:1.



