I lack the art gene. While other people can spend hours in a single room at the Met or MOMA, after five minutes I'm thinking about ways to light myself on fire. I just don't get art.
But I do get chemistry, and as we speak, there is an Andy Warhol exhibit at the Whitney Museum of American Art, which is a seriously hot ticket (I'd rather jam fishhooks in my eye). And these two converged when I read about some of the techniques that Warhol used, like peeing on paintings. And inviting others to do the same. This is not as crazy as it sounds ...
- Other artists have done far worse
- So has Warhol (1). Don't read this if you have a sensitive stomach.
- The chemistry makes perfect sense
- We all have to pee somewhere
So, before I piss away too much of your time, let's have a little lesson on "P-Chem." (2)
In 1977 Warhol put P-Chem to good use. He painted Oxidation Painting (in Twelve Parts) with copper paint, which (not surprisingly) looks like copper. That is until he and his guests whipped out (sorry) an acidic oxidizing solution. Then it turned into this.
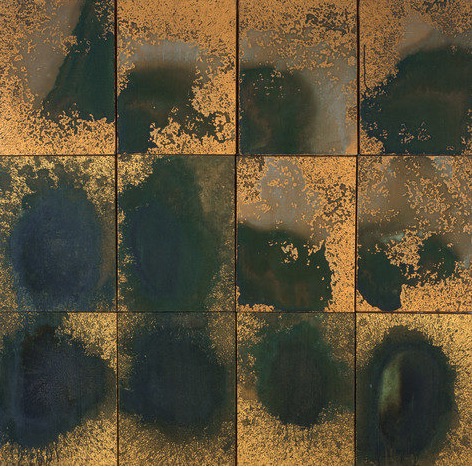
Oxidation Painting (in Twelve Parts), 1978. Photo: The Andy Warhol Foundation for the Visual Arts, Inc.
But Warhol didn't quite get chemistry...
It was just copper paint and you would wonder sometimes why it did turn green and sometimes it didn’t. It would just turn black or something. I don’t know what made it do that.
ANDY WARHOL IN MARK FRANCIS, ANDY WARHOL: 1956-86, MIRROR OF HIS TIME, 1996
So, what's with all the colors (not to mention highly variable quantity and aim)? Chemistry explains it all (not the aim). I will spare you the term "electromotive force potential of metals" and just call it the "relative reactivity of metals with acid." For reasons you don't want to know (it's based on the position of the metal on the periodic table), there is a wide range of reactivity of different metals with a water solution of a strong acid (e.g, hydrochloric, sulfuric). The figure below is divided into four somewhat subjective classes with the most reactive metals on top and the least reactive on the bottom. The red hatch line is not subjective. No metals below the line will react with hydrochloric acid; it is energetically impossible (3).
Since this article is about the uric acid in urine reacting with the copper paint isn't it odd that copper sits below the red line? If it doesn't react with acid then why are all these Greenwich Village-types giving golden showers to canvases? It's because there is more going on - oxidation.
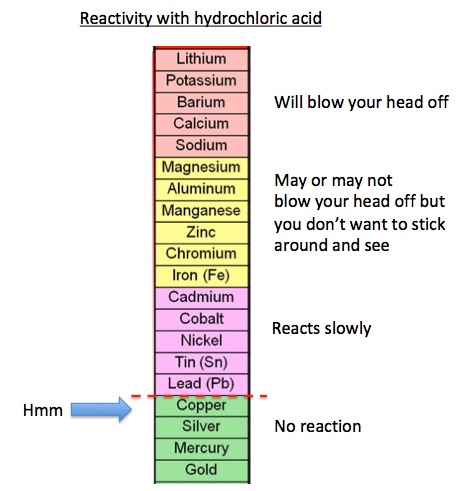
Relative reactivity of metals. Source: Chemistry Land
Copper may not react with acid but it certainly does with oxygen to give copper oxide, otherwise, this wouldn't have happened (See Copper: A Seriously Cool Element — Especially If You Like Colors And Money).

Verdigris. Photos: Daily Mail, Wikipedia Commons
Hold on! Copper can exist in two oxidation states, Cu+1(cuprous oxide) and Cu+2 (cupric oxide). But neither of these looks green. So what's going on?
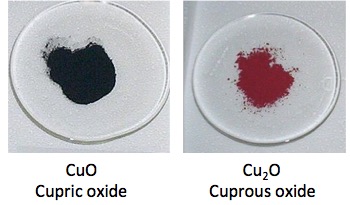
Copper (II) and Copper (I) oxides. Neither is green.
That's where the uric acid comes in. Unlike copper metal, copper oxides react with (and dissolve in) acid. Since uric acid is slightly acidic and urine is full of sodium chloride there will be a small amount of hydrochloric acid present. Cupric (copper (II) oxide will react to give cupric chloride. Cuprous (copper (I)) oxide will react to give cuprous chloride. This is what they look like:

(Left) Copper (I) chloride (Right) Copper (II) chloride.
OK, that explains the green (and the blue if you look hard enough, middle row, left) in the painting and the color of the Statue of Liberty (4).
Here's why people don't go into chemistry. Below are different photos of copper (I) chloride and copper (II) chloride. The same chemicals. But they sure don't look like the pretty ones above. The reason is that copper salts may either incorporate molecules of water within the crystals (hydrates) or not (anhydrous). And the color of the two forms is very different, even though they are the same chemicals.

(Left) Anhydrous cupric (copper (I)) chloride (Center) Anhydrous cuprous (copper (II)) chloride (Right) Dude couldn't handle chemistry.
Put all of this together and you may have:
1. Enough colors to analyze Oxidation Painting (and pretty much every other painting on earth)
2. The urge to drop acid. And why not? Andy Warhol did.

So, I guess there are a few lessons here.
- Chemistry is cool but can also make you crazy.
- Andy Warhol was worth $220 million at the time he died and I'm eating cat food.
- So, it is much better to pee on a painting than to try to explain what the pee did to the painting.
This realization has me severely pissed off. It's 6:30 and I'm still here writing this idiocy. Enough. I'm going home for dinner.

NOTES:
(1) Maybe not, but (prepare yourself) Warhol also ejaculated on paintings. Perhaps a contest is called for. Can you alter the name of a Warhol painting to reflect this vile bit of information? The more tasteless the better. Betcha I win.
(2) Bad pun aside, there really is something called P-Chem is short, which is short for physical chemistry. It includes charming subjects like quantum mechanics and thermodynamics, but it's mostly (shudder) math, especially calculus. Whatever that it. P-Chem is the personification of evil and has been the downfall of many chemistry grad students.
(3) It is no accident that some of the metals at the bottom are used in jewelry. Silver is almost unreactive (except for oxygen), gold and platinum are inert.
(4) It's not that simple. The colors of the statue come from other forms of copper (I) and (II) plus other stuff mixed in: a mixture of three minerals: Brochantite [Cu4SO4(OH)6] (green), Malachite [Cu2CO3(OH)2] (green), and Azurite [Cu3(CO3)2(OH)2] (blue), each formed from the environmental assault by oxygen, water, salt, and hydrogen sulfide.
(5) In reality, it's even worse. Uric acid is both an oxidant and an antioxidant, which means that it can convert copper (I) to (II) and back the other way too. Green to blue, blue to green.




