Coupla questions... What is the difference between the pennies below? And why does the penny on the left look like an albino? Hint: It has to do with the historical significance of the year in which it was minted. Answers will be forthcoming, thus ensuring that you will have to read this wretched piece to the end.
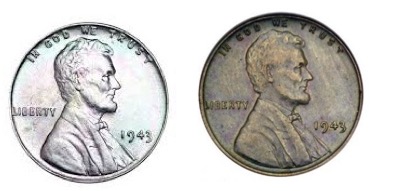
What's up with these two? Photo: Wikipedia Commons
MINERALS
Unlike nasty elements, such as fluorine (1) and mercury, (2) copper is one of the "elemental good guys." Copper metal has a number of unique properties that make it so important. Chemists routinely use copper compounds, and the colors of these and some copper-based minerals can be stunning.

Selected copper-based minerals Source: Dark Matrix Minerals, Wikipedia Commons, Geology.com
The most commonly used copper salt is copper sulfate. It is well known for its ability to form beautiful crystals, which makes it a staple of introductory chemistry labs.

COLOR CHANGES WITH OXIDATION STATE
Copper exists in three oxidation states: Cu0 (elemental copper), Cu+1 (cuprous), Cu+2 (cupric). Cu+2 undergoes an interesting reaction with several basic nitrogen-containing chemicals, for example, ammonia. When ammonia is added to a solution of copper (II) sulfate, it forms a complex [Cu(NH3)4]+2 in which four ammonia molecules bind to copper creating a deep blue-to-violet color.

(Left, copper color changes with oxidation state, (World Metals LLC). Right, copper ammonia complex, Fine Art America)
COLOR CHANGES FROM ENVIRONMENTAL CONDITIONS
The Statue of Liberty is made of steel coated with copper. With time and exposure to oxygen, copper corrodes forming a patina called verdigris. But copper oxide is black, so how did copper plus air turn green?

Photos: Daily Mail, Wikipedia Commons
The answer is that the green isn't copper oxide, but rather a mixture of three minerals: Brochantite [Cu4SO4(OH)6] (green), Malachite [Cu2CO3(OH)2] (green), and Azurite [Cu3(CO3)2(OH)2] (blue), each formed from the environmental assault by oxygen, water, salt, and hydrogen sulfide.
MISCELLANEOUS INTERESTING FACTS
- Copper, which binds tightly to oxygen, can replace the iron in hemoglobin—the carrier of oxygen in most living creatures— in the blood of some invertebrates, such as horseshoe crabs. The copper equivalent to hemoglobin is called hemocyanin. The blood of horseshoe crabs can be either blue or colorless, depending on whether it is oxygen-rich.
- Eighty percent of all of the copper ever mined has been recycled.
- Gold contains copper. In the absence of added copper, gold is so soft that you can mold it with your hands or bite it.
- Some sculptors pee on copper to give it a patina. Sculptors are weird.
OK, since you endured this whole thing, here are the answers about the pennies:
- The 1943 penny on the left is the only steel coin ever minted in the US. Pennies were made of steel during that year because of a copper shortage during World War II.
- This was done only once—in 1943
- A 1943 steel penny in perfect condition is worth about two bucks.
- The mint screwed up and minted a small number of 1943 copper pennies. The one on the right is worth about $80,000.
- You will not find one of these in an old jar of coins. Don't bother looking.
- The only other coin consisting of significant amounts of copper at that time was the nickel. All coins of higher denominations were 90% silver and 10% copper. The last circulating silver coins were minted in 1964.
- Before (and after) the war, nickels contained 75% copper and 25% nickel.
- In order to conserve copper, the composition of 1942-1945 nickels was also changed, but they were not made from steel. Instead, the composition of the "War Nickels" was 35% silver, 56% copper, and 9% manganese. They are worth between $1 and $700, depending on the condition and where they were minted (3). The silver nickels had a slightly different design on the back of the coin to denote that it was silver.

1943 silver ("War") nickel (left, center) and normal 1950 nickel (right). Note the difference in the color of the metals. The letters in red circles are called mint marks, which indicate where the coin was made. The War Nickels had their mint marks above Monticello on the obverse of the coin. All other nickels have them (and good luck seeing it) to the right of the building. Common mint marks are P (Philadelphia), D (Denver) and S (San Francisco). Rare mint marks include O (New Orleans) and CC (Carson City). These have not been used for many years.
That's about it. Any questions? A penny for your thoughts. Or not. Pennies minted since 1982 contain very little copper. They are now 97.5% zinc and only 2.5% copper. The value of the copper metal contained in an all-copper penny, if melted down, would two cents. Copper pennies would disappear from circulation overnight.
Notes:
(1) See: Fluorine: The Element From Hell
(2) See: Two Drops Of Death: Dimethylmercury
(3) Although this may seem silly to people who don't collect coins, the significance of the tiny mint market is anything but tiny. Coin value is based on condition and rarity. Some mints have made very few coins over the years, San Francisco (S) being one of them. A 1893 silver dollar in very good condition minted in Philadelphia is worth about $1500. But a 1893-S dollar in the same condition sells for $250,000.




