
How does Sirius XM Radio turn into a lesson on analytical chemistry? Last week I'm in my car and "California Dreaming" started playing. One of my favorites but I still can't get the damn music out of my head.
"All the leaves are brown
And the sky is gray
I've been for a walk
On a winter's day"
John and Michelle Phillips, The Mamas and the Papas, 1965
Although the four-part harmonies are beautiful, the song was botanically inaccurate; all the leaves weren't brown. There are still some fall colors around here. Which reminded me of a cute experiment I ran a long time ago in organic lab – the separation of the colored components of green leaves by a process called thin-layer chromatography (TLC).
TLC is the most common analytical technique used by synthetic organic chemists (1). I must have run 100,000 of them during my time in lab. TLC is so widely used because it is a (really) simple, powerful tool that can be used in a number of ways. You can use it to monitor the progress of a chemical reaction (decide whether it is finished), to get a rough idea of the purity of a chemical, or to see how many components are present in a mixture of chemicals. Here's how it works:
Glass plates, typically 1x3" are coated with silica gel, a white, freely-flowing powder that looks like bleached flour. Silica is (sort of) a form of finely ground up sand. The plates are manufactured with a binder (gypsum) mixed in to hold the silica to the plate.

Silica gel TLC plates, front and back.
TLC works because different chemicals have different affinities for the silica, therefore they will migrate up the plate at different rates. The plate is partially immersed in a solvent, commonly a mixture of ethyl acetate and hexane. It looks like this:
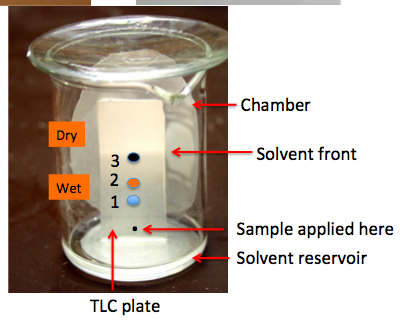
Figure 1. Depiction of thin-layer chromatography. 1) The developing solvent is put into a jar or chamber (a beaker with a watch glass top is used here). 2) A tiny amount of sample is applied to the plate using a capillary tube (black dot). This spot is called the origin. 3) The plate is then placed into the solvent, which (by capillary action) slowly moves up the plate - just as paper towels and sponges work. 4) When the solvent front reaches near the top of the silica, the plate is removed. 4) The components are visualized (2).
In Figure 1 we see that the sample was a mixture of three compounds. Component 1 is the most polar (see below); It adheres to the plate more strongly and stays close to the origin. Component 3 is non-polar; it moves with the solvent front. Compound 2 is somewhere in-between in terms of polarity. (This is a drawing; a real plate doesn't look this good. The spots aren't often perfect ovals or colored, and they don't separate this well from each other.)
Although most green leaves will work, spinach works especially well.
Spinach is ground up using a mortar and pestle with a little solvent present to extract the various chemicals in the leaf. Below, the sample is applied in a thin line instead of a dot. It works the same way. When the plate is put in the developing chamber cool things happen. Seven different components separate beautifully, forming colored bands, each representing a chemical in spinach (3).
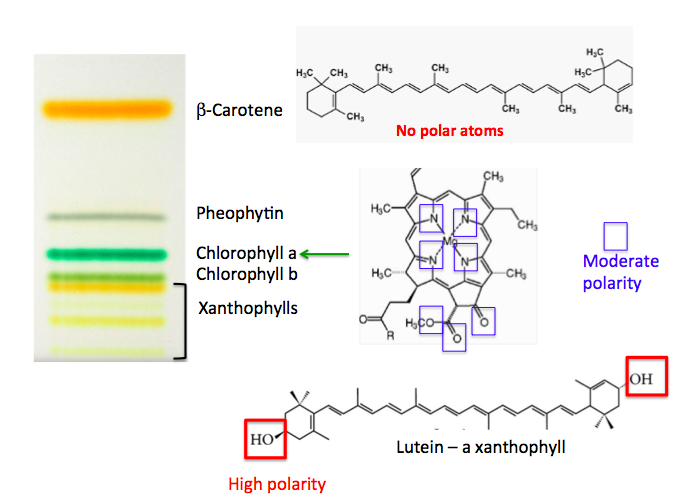
The order of the bands is not random. It tells us about the properties of the chemicals, specifically, how polar they are. ß-Carotene is the yellow band at the top. It should be. Note that it is composed entirely of carbon and hydrogen, just like oil, gasoline, kerosene. There are no heteroatoms – a term organic chemists use to describe atoms other than carbon and hydrogen (typically oxygen, nitrogen, and sulfur). Heteroatoms make a compound more polar and it will bind better to the silica. Chlorophyll has a bunch of heteroatoms and does not move as far from the origin as ß-carotene. But more doesn't mean better.
Lutein contains only two oxygen atoms, but they are bound to hydrogen (this is called a hydroxyl (O-H) group)). Hydroxyl groups are polar for the same reason that water is.
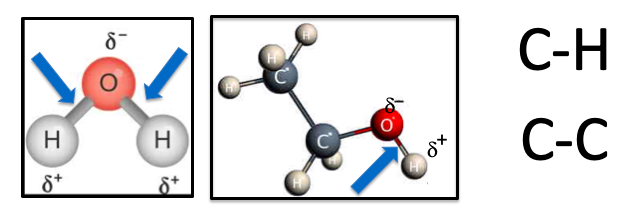
Polarity in chemistry means separation of charge. The bonds (blue arrows) in water (Left) and ethanol (Right) are polar because oxygen atoms are highly electronegative – they really like electrons. So the oxygen atom "sucks" some of the electron density from the O-H bond causing it to become polarized, leaving a slightly positive hydrogen atom (delta stands for "slightly") and a slightly negative oxygen atom. By contrast, neither carbon nor hydrogen really give a #### about electrons, so they are perfectly content to share them equally. These bonds are non-polar. This is the basis of "like dissolves like" – the reason why water and alcohol mix and water and oil do not, and also why you can see the colors of spinach.
Well, it's "Monday Monday" night, which means it's the start of the fantasy basketball week. So I need to go watch the hideous team that I picked torture me. Just like I did to you.
NOTES:
(1) Synthetic organic chemistry is a technique for converting one chemical compound to another. This is precisely what Walter White did when he converted Sudafed to methamphetamine.
(2) Most organic chemicals are white solids or colorless liquids so there are a number of ways to visualize them, like staining the plate with iodine or shining a UV light on it.
(3) There are many more chemical compounds present in spinach. Some are not colored so they can't be seen. Some are present in amounts too small to see. And there can be bands within each band that just happened to run up the plate the same distance (co-elution).



