There are several coronavirus vaccines that are currently in Phase 3 clinical trials. For vaccines, this final experimental stage requires tens of thousands of people in order to properly assess efficacy and safety. Among other things, such a large sample size is important for detecting rare but dangerous side effects.
It was just reported that AstraZeneca, a British pharmaceutical firm collaborating with the University of Oxford on a COVID vaccine, has paused its Phase 3 trial because one volunteer has developed an "unexplained illness," according to Stat. No other details are available. So, what could it be?
Vaccine Side Effects
Most vaccine side effects are familiar to anyone who has ever gotten a jab: Sore arm, swelling or redness at the injection site, fatigue, and maybe a fever. Collectively known as reactogenicity, these are all classic signs of an inflammatory response, which is good, because that's exactly what a vaccine is trying to elicit. The hope is that, ultimately, the immune system "remembers" what it responded to so that it is ready to attack an infectious microbe if a person is exposed.
But tinkering with the immune system can be dangerous. Sometimes, the antibodies produced in response to the vaccine actually make an infection worse, a phenomenon known as vaccine-enhanced disease. In other cases, the antibodies elicited start attacking the patient's own body, which is an autoimmune disorder. Most likely, the volunteer who is thought to be experiencing a serious adverse reaction to the coronavirus vaccine is suffering from one of these two conditions.
We know from previous research on coronaviruses, including the original SARS virus (now called SARS-CoV-1), that vaccines can make the disease worse. So one possibility is that the patient was in the experimental group (which means he or she would have received a vaccine rather than a placebo) and was subsequently exposed to the virus while going about daily life.
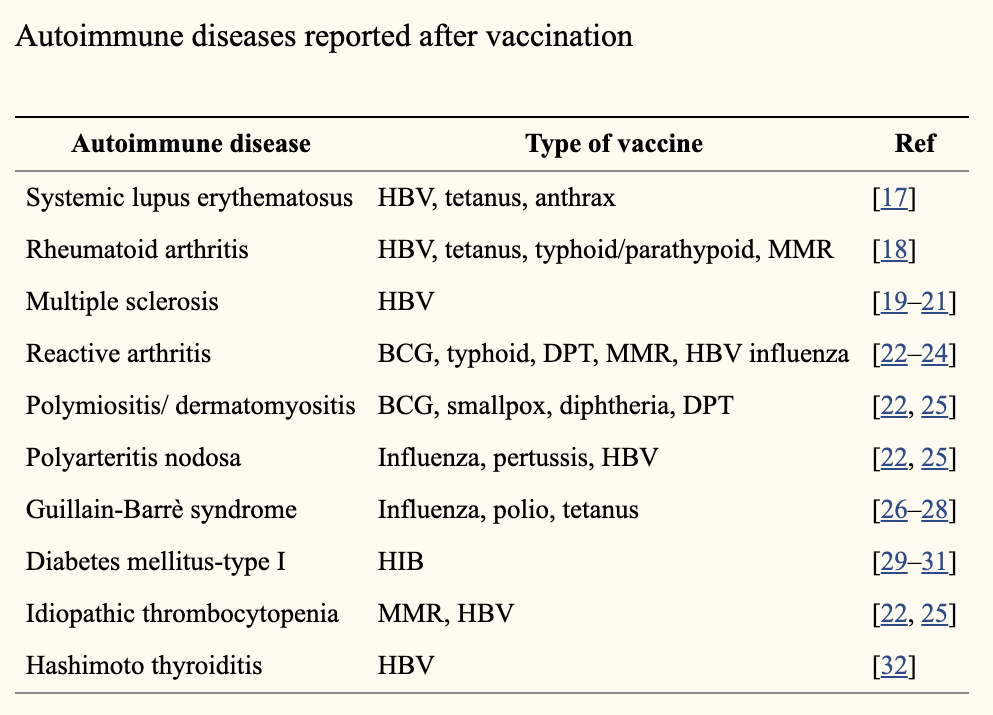 The second possibility, which is probably more likely, is that the volunteer developed an autoimmune response. A review article published in the EPMA Journal lists several reported autoimmune conditions following vaccination. (See chart.) Guillain-Barré syndrome (GBS) caused by the flu vaccine is one of the more infamous examples of adverse reactions, so let's discuss it in a little more detail.
The second possibility, which is probably more likely, is that the volunteer developed an autoimmune response. A review article published in the EPMA Journal lists several reported autoimmune conditions following vaccination. (See chart.) Guillain-Barré syndrome (GBS) caused by the flu vaccine is one of the more infamous examples of adverse reactions, so let's discuss it in a little more detail.
GBS can be triggered both by natural infections and vaccines. In fact, according to the Children's Hospital of Philadelphia, GBS is about 17 times more likely to be caused by an actual influenza infection rather than the flu shot. (Thus, the flu vaccine could be seen as preventing GBS.)
Regardless of how it originates, GBS causes a creeping paralysis on both sides of the body that begins at the ends of the limbs (hands and feet) and moves "upward." While the exact details are unknown, the symptoms of GBS result from immunologically mediated nerve damage. Specifically, the protective covering of nerves, known as the myelin sheath, suffers injury. That causes the nerves to malfunction, resulting in paralysis. Most people recover; some have permanent nerve damage.
Why would this happen to some people but not (most) others? It probably comes down to genetics. That is, some people may be more prone to autoimmune disease, and all that's needed to trigger it is exposure to a particular antigen. Biomedical science has not advanced to the point that those with underlying genetic conditions could be identified in advance.
Is the COVID Vaccine Responsible for the Volunteer's Adverse Event?
There is, of course, a third possibility: The volunteer was in the control (placebo) group, and his or her adverse reaction had nothing to do with the vaccine. Given that the trial was paused, this is probably unlikely. But so little information is currently available that it can't be ruled out.
It should be kept in mind that adverse events should not necessarily rule out a mass vaccination campaign. Once all the data are collected, scientists will need to determine if the risk of COVID-19 is greater or less than the risk posed by the vaccine. Most likely, the risk of the disease will be greater than the risk of side effects. But we don't know for sure. That's why we do the clinical trials.




