Some of you screwballs out there have been complaining that I'm not giving enough chemistry lessons. No accounting for taste. If you'd asked me a few years ago when I first started writing these miserable things I would have doubted that my own family would bother to read them, but it turns out that they've become strangely popular.
So, let's do another.
THE MORE OR LESS SORDID HISTORY OF HEROIN
Here's the first journal article on the synthesis of heroin. It is long and complex, despite the fact that the conversion of morphine to heroin is (now) a trivial reaction that can be done in minutes.
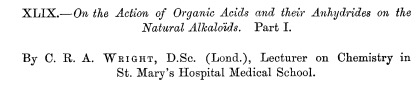
The title of the first reported synthesis of heroin. Wright, C. R. A.: On the action of organic acids and their anhydrides on the natural alkaloids, Journal of Chemical Society, 1874, 12, 1031. The paper contains no chemical structures, only molecular formulas. Does anyone know why? (1)
Source: Journal of the Chemical Society
OK, it's time...

Making Heroin is Easy Peasy. But it wasn't in 1874
Our progress in fighting pain hasn't progressed much since 1874, but organic chemistry has. It took four pages of experimental details for Wright to describe the procedure for the synthesis, purification, and analysis of the heroin he made. All of this for one very simple reaction...
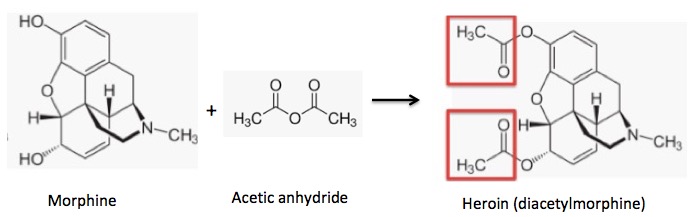
Acetylation of morphine: Two hydroxyl groups react with acetic anhydride to form two acetate esters (red boxes) (heroin).
By contrast, the synthesis of aspirin from salicylic acid – an almost identical chemical reaction – takes 10 minutes (plus a little purification time) in an experiment suited for high school or organic 101 students.
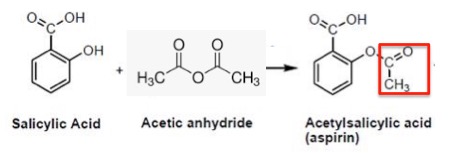
Salicylic acid contains one hydroxyl group, which is easily acetylated to form aspirin. The acetyl group is shown in the red box.
See how similar the two reactions are?
ASPIRIN VS. HEROIN
In the 1890s Felix Hoffman, a German chemist working for Bayer was acetylating everything in sight to examine the change in properties of chemicals/drugs when hydroxyl groups are converted to acetate esters. Although Hoffman wasn't the first person to synthesize either aspirin or heroin, he did make both for Bayer, which sold both of them...at the same time... and in the same ad(!).

A Bayer ad, date unknown, selling both aspirin and heroin. Image: Bomb Magazine. At the time that the article was written (1982), no one had ever heard of fentanyl. This story would read very differently now.
You've withstood the DCLFHTM so what is your reward?
A BIOCHEMISTRY LESSON FROM HELL!

Why do the acetyl groups found in heroin and aspirin give the drugs enhanced properties compared to the hydroxyl versions, morphine, and salicylic acid, respectively? The acetyl groups make a very big difference in the potency of the drugs in each case, but for different reasons.
ASPIRIN
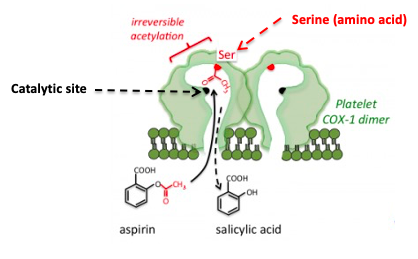
Figure 1: The inhibition of COX by aspirin. Aspirin forms a covalent bond to a serine group near the active site, which prevents the normal substrate from entering the channel to the catalytic (active) site of the enzyme. Source: Tulane University
Aspirin and other NSAIDs work by inhibiting a critical enzyme called cyclooxygenase (COX), which catalyzes the conversion of a ubiquitous biomolecule, arachidonic acid (AA) into prostaglandins and thromboxanes – extremely potent hormones that have multiple functions throughout the body, including, pain and inflammation. As seen in Figure1, aspirin (4) fits into the channel in COX that leads to the catalytic (active) site (black dot).
Once there, the acetyl group, which is chemically reactive, reacts irreversibly with the serine – another way of saying that aspirin transfers the acetyl group to COX forming a covalent bond. Once the serine group of the enzyme has been acetylated things grind to a halt; the enzyme is inactivated. Arachidonic acid can no longer reach the catalytic site on COX so it cannot be converted to prostaglandins and thromboaxnes. This explains why aspirin, ibuprofen, etc. treat pain, fever, and inflammation (and also why they screw up your stomach.)
MAKING SENSE OF MORPHINE AND HEROIN
The function of the acetyl groups in heroin is completely different than that in aspirin. In this case, they are merely delivery devices - sort of molecular UPS trucks.
The two hydroxyl groups in morphine make the molecule hydrophilic (water-loving – the opposite of lipophilic – fat-loving). Lipophilic molecules are usually better at penetrating cells (and also getting into the brain) because they can pass more easily through cell membranes. Lipophilicity is measured or calculated (2) and expressed by a unit called logP, the higher the value the more lipophilic the molecule. (P is called the partition coefficient. Don't ask.) All you need to know is this:
A drug targeting the central nervous system (CNS) should ideally have a logP value around 2
Source: ACD Labs
Now, let's look at the logP values for morphine, heroin, and its primary metabolite, 6-monoacetylmorphine. It should not be surprising that each time an acetyl group replaces a hydroxyl group the molecule becomes more lipophilic. (For the chemical structure of these drugs see Figure 2 below.)
Morphine (two hydroxyl group) - 0.8
6-Monoacetylmorphine (one hydroxy group) - 1.3
Heroin (no hydroxyl groups) - 1.5
Sorry to interrupt your coma, but there is a bit more information you need to know in order to understand why heroin behaves like heroin – metabolism.
Although heroin's logP is close to 2 – the ideal value for CNS drugs – the difference between it and morphine, 0.7 doesn't seem like a big deal. But keep in mind that a 0.7 log10 unit difference means that heroin is 6.5-times more lipophilic than morphine, so it gets to the brain more easily (this explains the "heroin rush").
Heroin's two acetyl groups are metabolized at different rates and at different places in the body. This what makes heroin such an effective and deadly drug.
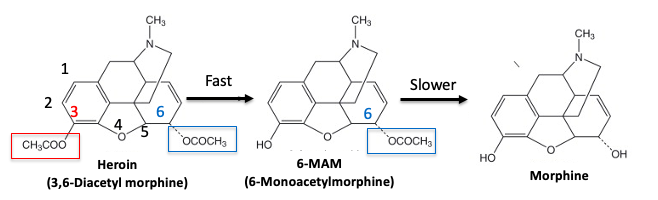
Figure 2. Metabolism of heroin. Original Source: Science Direct
Step 1. The acetyl group at the 3 position of heroin (3) (red box) is very reactive. It is cleaved by esterase enzymes in the blood in a few minutes, forming 6-monoacetylmorphine (6-MAM). This reaction occurs so quickly that heroin doesn't even reach the brain. And if it did, it wouldn't matter because heroin itself is not an opioid agonist.
Step 2. 6-MAM is also deacetylated in the blood, forming morphine, but not so fast that it cannot reach the brain. It does so very quickly. In fact, 6-MAM itself is a mu-opioid agonist and this is probably why heroin packs more of a punch than morphine. Additionally, 6-MAM is deacetylated by the brain to give morphine.
It is not completely clear whether the "heroin rush" is due to 6-MAM itself, the rapid delivery of 6-MAM to the brain where it is converted to morphine, or both. But it doesn't really matter. Heroin is a very effective pro-drug of morphine (and/or 6-MAM).
It's amazing, in a twisted sort of way, that heroin, the scourge of mankind (at least until fentanyl came along) is a quintessential example of the power of pro-drugs.
Chemistry lesson over. Biochemistry lesson over.
You can wake up now.
NOTES:
(1) At this time chemical structures were unknown. Chemists could not determine how the atoms of a molecule were connected, only the molecular formula of that molecule.
(2) Virtually all the numbers given for logP are calculated, not measured. To do this, it would take an eternity and cause chemists to be hurling themselves into the Kilauea Volcano. I have no idea how this calculation is done and am perfectly content to keep it that way.
(3) Why is that called the 3-position? Although there are rules for assigning numbers to molecules for the purpose of naming them I'd rather drink nitric acid than go back and relearn them. Nomenclature makes organic chemists (more) crazy.
(4) Aspirin is the only NSAID to inhibit COX by forming a covalent bond. The others do so by tightly (but reversibly) binding to the enzyme.