"Clinical trial" is just a nice-sounding term for "human experiment." We don't like to use that term because it carries a lot of baggage, like the torturous and murderous "experiments" conducted by Nazi doctor Josef Mengele.
But if we're being completely honest, when scientists and doctors perform clinical trials, they are conducting experiments on human volunteers. As a result, there are a lot of laws and regulations that govern the ethics of clinical trials.
For instance, it is unethical to just test any random drug in a patient to see if it cures a disease. There has to be a rational, scientific basis for the chosen drug. Even in the case of a desperate, terminally ill patient, it is unethical to "try anything" because the wrong drug could make the suffering or pain even worse.
Another key feature of an ethical clinical trial is to provide a treatment (rather than a placebo) if one actually exists for the disease under study. (In these sorts of clinical trials, the experimental treatment is compared to the "standard" treatment to determine if it is superior.) In the infamous Tuskegee experiment, this did not occur: Antibiotics were purposefully withheld from Black Americans so that scientists could observe the effects of syphilis on the body. This was a horrible blight on the history of medical research in our country.
Are Most Psoriasis Clinical Trials Unethical?
The question of placebo ethics becomes a bit murkier when the disease under investigation isn't lethal or insanely painful. Ethics are also complicated if the standard treatment is dangerous in some way. In these instances, it isn't clear if a placebo or the standard treatment is the more ethical option.
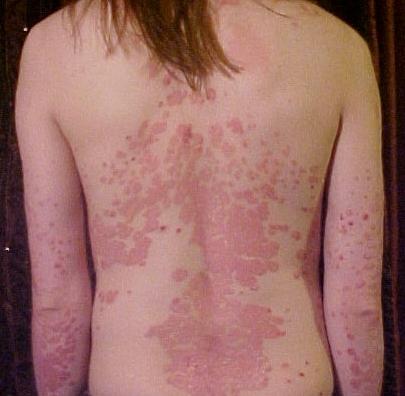
Take psoriasis, for instance. This disease is thought to be autoimmune, and its characteristic manifestation is red skin patches with scales. For some people, the disease is itchy or painful; for others, it's just ugly. Treatments exist, but because they are immunosuppressants, they can have serious side effects such as increasing the likelihood of infection.
But, because successful treatments do exist for psoriasis, is it unethical to withhold them from volunteers in clinical trials for new psoriasis drugs? A group of French researchers believes the answer is yes, and they have published a paper showing that 75% of psoriasis clinical trials still use a placebo. (Note: Only the abstract is available.) The authors conclude that this needs to be improved.
Are they right? It depends. If the volunteers are experiencing substantial pain because of their psoriasis (e.g., a form called psoriatic arthritis, which causes joint pain), a placebo may indeed be unethical. But if patients are not experiencing pain, then the standard treatment may be unethical if it increases the risk of infection.
As is so often the case, such as with placebos in coronavirus vaccine trials, the ethics aren't entirely clear.