
It wasn't surprising when dexamethasone was found to be useful in treating severe COVID disease. One of the many puzzling symptoms of this "new" disease was an overreactive immune system and the inflammation it caused. And if there's one thing steroids can do it's reducing inflammation. Although dexamethasone, a 60-year old drug, was effective in calming COVID it's very likely that other antiinflammatory steroids like prednisone or prednisolone will also be effective. Look at the similarity in chemical structures:
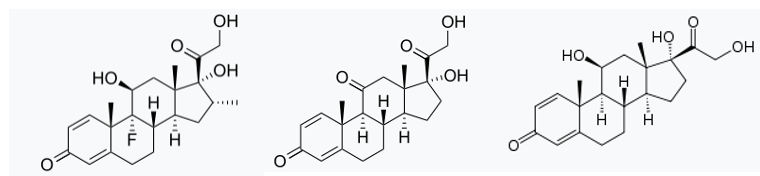
The chemical structures of dexamethasone (L), prednisone (C), and prednisolone (R) are very similar.
Budesonide to the rescue
There's a new "sheriff" in town. Its name is budesonide and in a small study at the University of Oxford, it was shown to be very effective in preventing severe COVID. If you're an asthmatic (like me) the name should sound familiar.
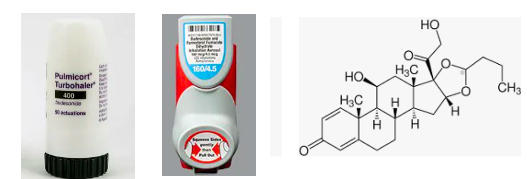
(L) Two (of many) budesonide containing inhalers. (R) The chemical structure of budesonide
A new study published online in The Lancet Respiratory Medicine shows that budesonide is effective in preventing severe COVID. Normally, this would be a snoozer – just another steroid working as expected. But there is a significant advantage to this therapy – the fact that the steroid is inhaled avoids most of the side effect of systemic steroids (2), which can be really nasty (See Prednisone: Satan's Little Helper).
The studies
A total of 146 participants in the town of Oxfordshire in the UK, all of whom met the eligibility requirements (1) for the trial, were divided into two equal groups. One group received standard care and the other got budesonide. The primary endpoint of the trial was whether the participants required an urgent care visit (related to COVID). The inhaled budesonide worked quite well. In the standard care group, 10 people required urgent care while in the budesonide group only one person needed it. Although the number of participants was low the results were statistically significant (p = 0.004).
Another advantage
Poorer countries are (not surprisingly) far from the top of the list of vaccine distribution. A drug that is effective, cheap, easy to use, and requires no refrigeration would be a game-changer in parts of the world that do not have the weapons needed to control COVID. A puff or two, perhaps prophylactically, could go a long way toward reducing the morbidity and mortality of the disease.
The great asthma mystery of 2020
Early on in the pandemic, a strange result was noted; asthma patients were underrepresented. In other words, asthma patients seemed to become seriously ill less often than expected, not more. In fact, the Lancet authors were inspired to conduct this study because of this perplexing finding:
"We selected this treatment intervention due to the unexpected observation of an under-representation of patients with asthma and COPD with severe COVID-19."
S. Ramakrishnan, et. al., "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial." The Lancet Respiratory Medicine, April 9, 2021. DOI:https://doi.org/10.1016/S2213-2600(21)00160-0
One working theory for the apparent asthma paradox is that asthmatics regularly take inhaled steroid powders, budesonide being one of them, so their lungs are prophylactically protected from the inflammation caused by COVID. This study supports (but does not prove) this theory, so the answer to "the great asthma mystery" remains elusive, although perhaps a little less so.
Meanwhile, I will keep inhaling my delicious steroids every day, just like I've been doing for many years. Can't hurt.
NOTES:
(1) Eligibility requirements. "Adults aged older than 18 years with symptoms of COVID-19 (new onset cough and fever or anosmia, or both) within 7 days were eligible for inclusion. Participants were excluded if they had recent use (within 7 days) of inhaled or systemic glucocorticoids or if they had a known allergy or contraindication to inhaled budesonide."
(2) Dexamethasone is given by IV or as a pill.



