I tried it. I got hooked. The damn game is fun.
This does not mean that things went well, or that there weren't negative consequences.
Nope.
There were.

Perhaps my racquet skills were a bit rusty, but at least I'm still fleet-footed enough to navigate the court gracefully. Someone even took a photo!

Obscure (and mostly useless) things about pickleball (Source: The Racket Life)
- The ball must be made of smooth molded material and a uniform color. It must have between 26 and 40 circular, evenly-spaced holes.
If ACSH ever goes out of business, I want to be a hole spacer quality control specialist.
- When the ball is dropped from a height of seventy-eight inches, it must bounce between thirty and thirty-four inches high.
But there is no mention of how far it must bounce when someone smashes it into your face.
- Playing with the right type of ball makes the game much more enjoyable, while playing with the wrong kind may leave players frustrated and disheartened.
Tell me about it...

It's real. Don't ask.
- The ball must have a hardness of between forty and fifty on the Durometer Shore D Scale.
Fortunately, I always carry one of them with me.

Image: Wikimedia Commons
The chemistry of pickleball materials
"Low-density polyethylene or LDPE (softer) is one of the types of plastic that pickleballs can be made of. Other types of plastics are High-Density Polyethylene (harder)."
I didn't know any of that, but here's the general chemical reaction for how all polyethylene is made:
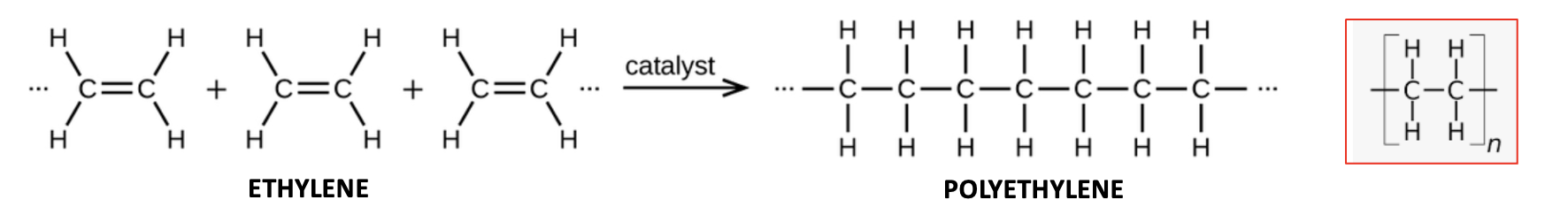
(Left) The polymerization of ethylene (the monomer) is promoted by certain metal catalysts, for example, titanium and chromium compounds or heat. (Right) A more compact representation of polyethylene (the polymer). The letter n represents the number of repeating CH2CH2 units, which can range from hundreds or thousands. Image: Wikipedia
What is the difference between LDPE and HDPE?
Who cares? Well, I do. If I'm going to have 26-40 evenly spaced holes rocketing at my nostrils I'm gonna choose low-density. Fortunately, this is the plastic used most frequently in pickleball.
The key here is the letter "D" – density, high and low; the more dense the harder. While the primary chemical reaction – polymerization of ethylene – is the same, the method of polymerization and the structure of the two classes are different.
High-Density Polyethylene (HDPE) is a linear ethylene polymer made using a Ziegler–Natta catalyst – a large group of metal salts that includes aluminum, titanium, zirconium, chromium, and others. Here's what HDPE looks like:
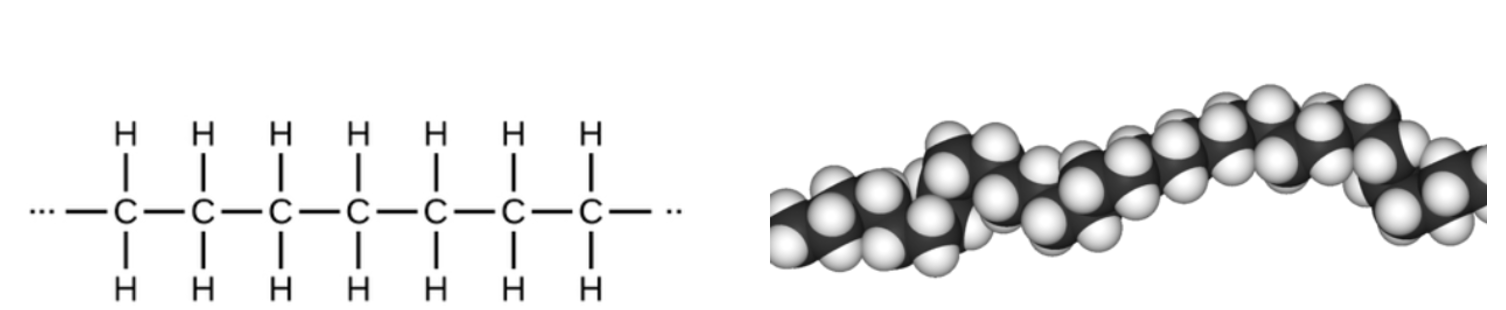
High-density polyethylene (HDPE) is an ethylene polymer shown as a simple chemical formula (left) and a space-filling model (right). The carbon atoms are black, and the hydrogens are white. Note that even though it curves HDPE is linear – a long continuous chain of CH2 units with no branching.
QUIZ: The is something with the same chemical formula (although a different n number) found at the pharmacy. You may have even had the pleasure of drinking it. What is it?
By contrast, LDPE is a branched polymer. The conditions to make LDPE from ethylene are different – heat and pressure.
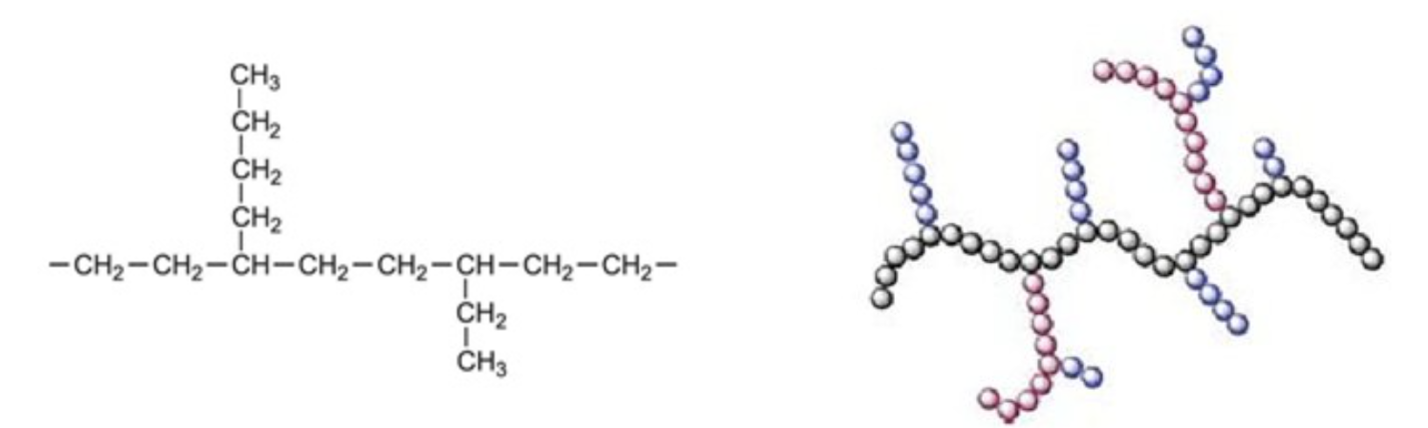
LDPE is a branched chain of CH2 units. (Left) Simple chemical formula. (Right) 3D representation showing random branches. Source: Journal of Elastomers & Plastics
What does this have to do with density?
Much like other organic chemicals, in linear polymers, the CH2 strands can get closer together (packing), resulting in a higher density. But in branched polymers, the branches get in the way of packing, which leaves more space between the carbon atoms, resulting in a lower density.
Here's a similar example using simple, low molecular-weight organic chemicals
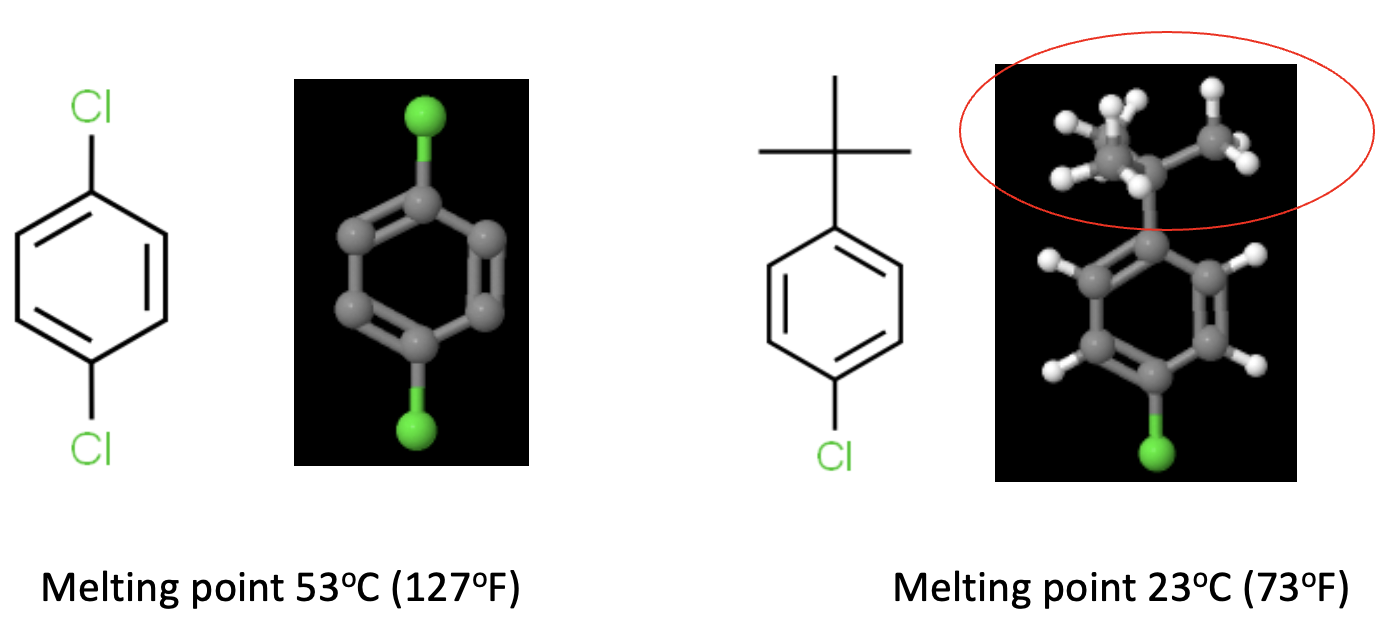
2D and 3D representations of two "similar" organic chemicals. Para-dichlorobenzene (left) is symmetrical and flat. Its melting point is 53oC (127oF). However, replacing one of the chlorine atoms with a bulky t-butyl group (red oval) has a profound effect. This chemical does not attain a crystalline form as easily and has a melting point of 23oC (73oF) and will melt in a warm room or in your hand.
All in all, it was a positive experience, but I have a long way to go before I'm playing at a more advanced level. In the meantime, I'm grateful that the other beginners have so graciously accepted me.

Fire Island Pickleball Club, Beginners Group. Images: Wikimedia Commons, Wikimedia Commons, Wikimedia Commons,



