I can hardly believe that it's been more than 10 years since I torched flibanserin (Addyi), aka "Female Viagra."
adverse effects
It is officially July, and that means something in the medical world. With the passage of one day, the first of the month exalts recent medical school graduates to the rank of intern.
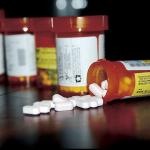
Medication mistakes outside of a health care facility are on the rise and resulting in serious outcomes—with home locations leading the pack.
Many people think they have a drug allergy, when in fact what they have is drug intolerance. According to the CDC, approximately 10% of all U.S.